Translate this page into:
What's new in cicatricial alopecia?
Correspondence Address:
Sunil Dogra
Department of Dermatology, Venereology and Leprology, Post Graduate Institute of Medical Education and Research, Chandigarh - 160 012
India
| How to cite this article: Dogra S, Sarangal R. What's new in cicatricial alopecia?. Indian J Dermatol Venereol Leprol 2013;79:576-590 |
Abstract
Scalp hairs complete the body self-image and patients with alopecia suffer from overt disfiguration, leading to psychosocial embarrassment and significant lack of self-esteem. Hence an early diagnosis and an aggressive treatment in the case of active hair loss are crucial in the management of scarring alopecia. This review presents a comprehensive study of newer theories in aetiopathogenesis, evolving diagnostic modalities and a step ladder approach in management of primary cicatricial alopecia.Introduction
Cicatricial alopecias (CAs) or scarring alopecias are a group of uncommon inflammatory hair loss disorders, which are characterized by permanent destruction of hair follicles. Clinically there is loss of visible follicular ostia in the scarring area, with or without epidermal atrophy and histologically there is absence of pilosebaceous structures which are replaced by fibrous tracts. [1],[2],[3],[4],[5] CAs are classified as primary cicatricial alopecia (PCA), secondary cicatricial alopecia (SCA), and developmental/hereditary CA.
PCA is caused by destructive inflammation of the hair follicle. This destruction is attributed to various etiologies, which are predominantly autoimmune processes. In this article, we aim to review newer theories in aetiopathogenesis, recently described clinical patterns, and advances in management of the PCA.
Epidemiology
Very few data on the epidemiology of CA is available in the published literature. One large retrospective study over 10 years showed the frequency of CA as 7.3% of all the hair loss cases. The majority of affected adults were females (2.6:1) and PCA was more common than SCA (4:1). Among causes of PCA, pseudopelade of Brocq (PPB) (40.6%) was most frequent followed by lichen planopilaris (LPP) (12.6%), and folliculitis decalvans (FD) (11.2%). [1]
In another retrospective study, 3.2% patients of hair disorders seen over 5 years had PCA, with majority cases characterized histopathologically by lymphocytic infiltrate (4:1). In this study, discoid lupus erythematosus (DLE) (33.9%) was the most common cause followed by PPB (24.1%) and LPP (22.3%). [2]
A recent questionnaire survey in UK, revealed incidence of PCA as 6.96 cases per 1000 new general dermatology referrals per year, equating to about 9.6 new cases per clinician per year. [3]
Classification
The causes of CA are broadly classified as primary, secondary, and hereditary or developmental defects. [4],[5],[6] In PCA, the hair follicles are the main targets of destructive inflammatory process resulting in irreversible hair loss from the affected site on scalp. The inflammatory cells targets and destroys the stem cells in the bulge area of hair follicles. [4] In SCA, the hair follicles are secondarily damaged as a result of more generalized destructive process within the skin, which ultimately destroys the hair follicle stem cell (HFSC)-based capacity for regeneration. [5] The causes of SCA are trauma (burns, radiation, traction), any infiltrative processes (morphoea, scleroderma, sarcoidosis, neoplasias), and infections (bacterial, fungal, viral, mycobacterial). [6]
Further, the working classification of PCA is based on the most representative pathological finding on scalp biopsies. PCAs are divided into subgroups depending upon the predominant inflammatory infiltrates, that is, lymphocytic, neutrophilic, and mixed [Table - 1], [Figure - 1], [Figure - 2], [Figure - 3], [Figure - 4], [Figure - 5], [Figure - 6], [Figure - 7], [Figure - 8], [Figure - 9] and [Figure - 10]. Chronic cutaneous lupus erythematosus (CCLE), LPP, classic PPB, central centrifugal cicatricial alopecia (CCCA), alopecia mucinosa (AM), and keratosis follicularis spinulosa decalvans (KFSD) are categorized under "lymphocytic" PCA. Frontal fibrosing alopecia (FFA) and Graham-Little-Piccardi (GLP) syndrome are considered as LPP variants. The neutrophilic PCA group comprises FD and dissecting cellulitis/folliculitis of scalp (perifolliculitis abscedens et suffodiens). Acne keloidalis nuchae (AK), acne necrotica (AN), and eruptive pustular dermatosis (EPD) are classified as "mixed" cell infiltrate PCA. In addition, nonspecific CA is defined as "idiopathic scarring with inconclusive clinical and histopathological findings". There have been debates whether this classification is satisfactory or not, however, it provides a practical and reasonable standard for clinical and basic studies thus has been widely used. [5],[6],[7]

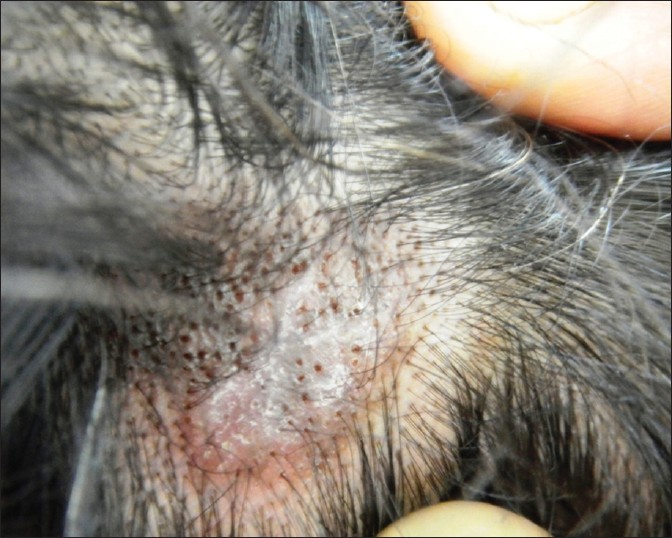 |
| Figure 1: Scalp DLE, showing erythematous atrophic plaque with central depigmentation, dilated follicular orifices, follicular plugging, and loss of follicular ostia |
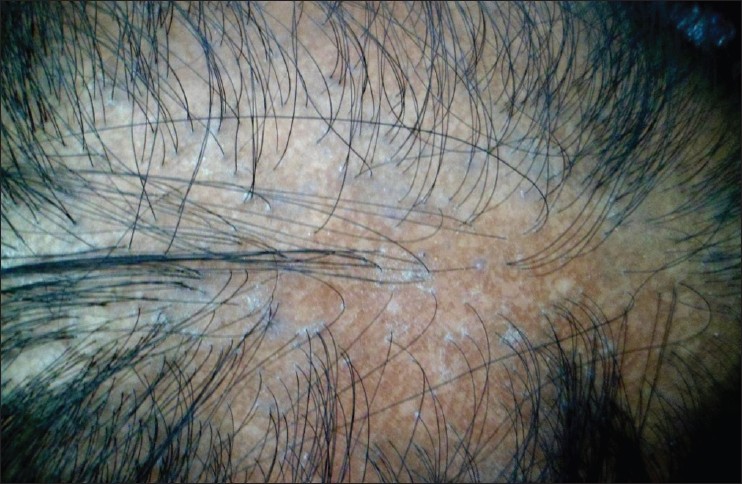 |
| Figure 2: Patch of LPP, showing violaceous, peri-follicular papules in the periphery and loss of follicular ostia, dyspigmentation in the center |
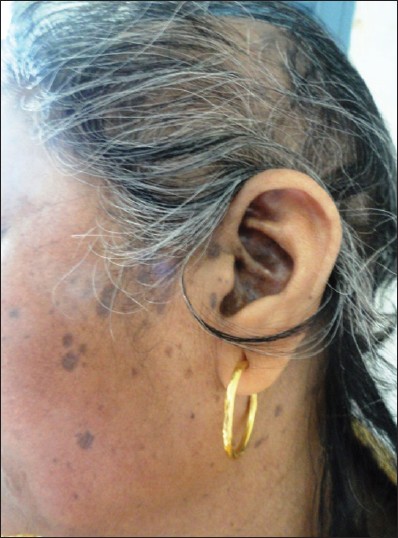 |
| Figure 3: Violaceous pigmented spots of LP on face along with scarring alopecia |
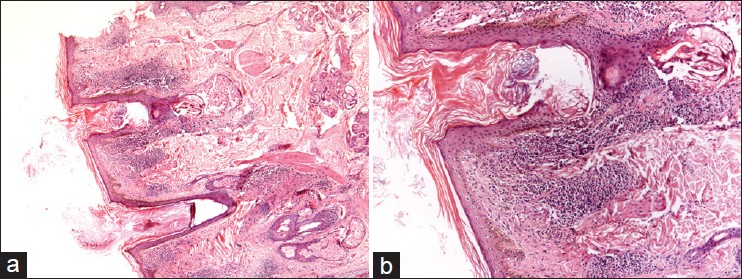 |
| Figure 4: (a) Microphotograph showing hyperkeratosis with peri-follicular inflammation and atrophy of sebaceous unit in a case of LPP (H and E, ×20), (b) Heavy lymphocytic infiltrate around dermoepidermal junction and peri-follicular area (H and E, ×40) |
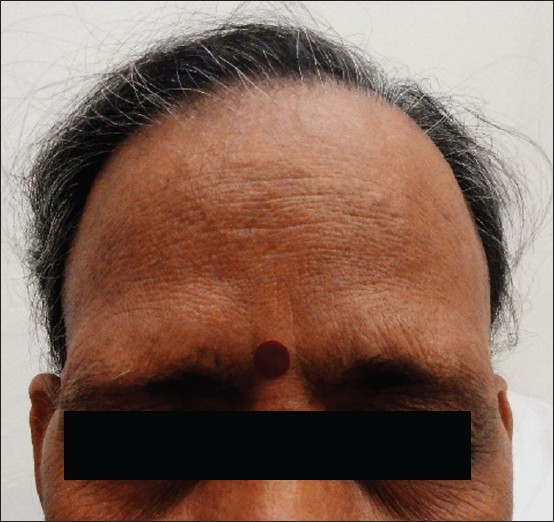 |
| Figure 5: Wide band of bald frontal scalp with bilateral symmetrical thinning of lateral eyebrows in postmenopausal women suffering from FFA |
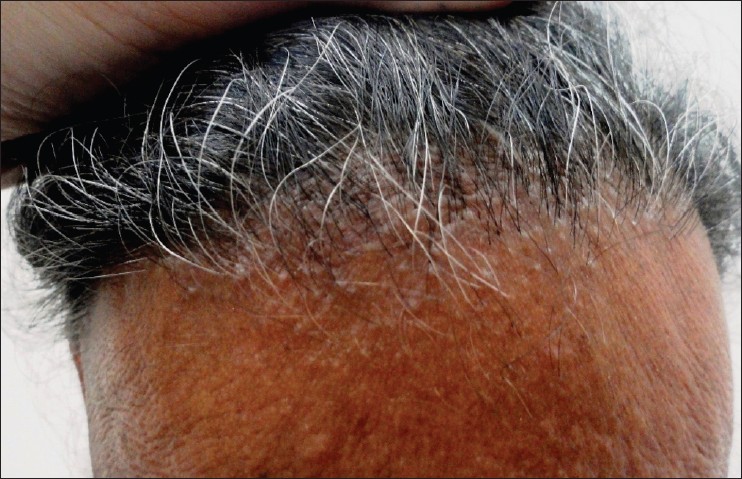 |
| Figure 6: Closer view of the frontal scalp of the same patient suffering from FFA, showing perifollicular erythematous papules at the hair margin (in the active stage of disease) |
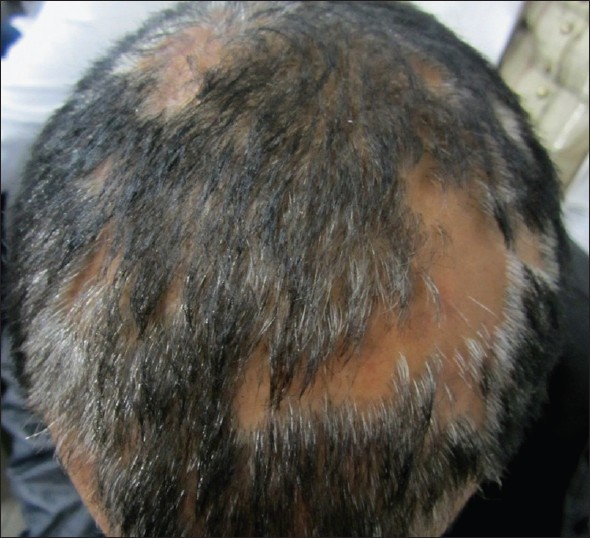 |
| Figure 7: Multifocal, skin-colored plaques of pseudopelade of Brocq with complete loss of follicular ostia and no signs of inflammation |
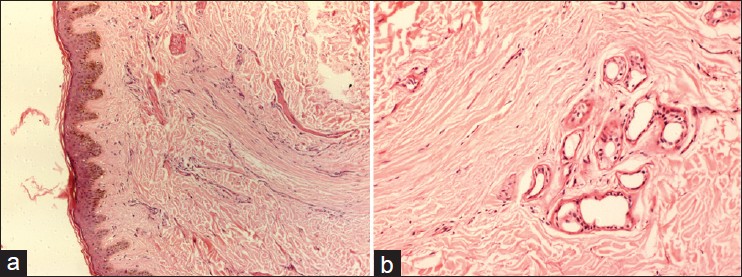 |
| Figure 8: (a and b) Microphotograph showing thinned out epidermis with total loss of hair follicles, replaced by collagen in a case of PPB (H and E, ×20) |
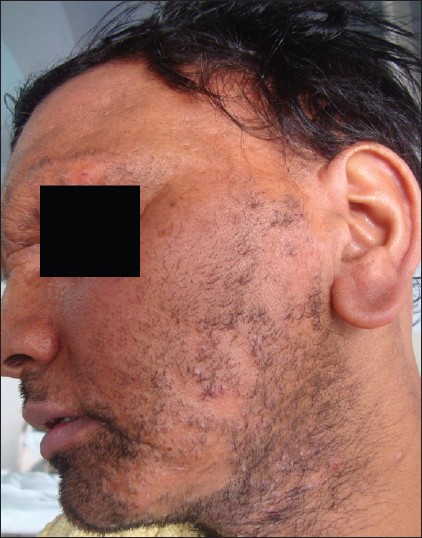 |
| Figure 9: Multiple scarring alopecia areas seen in beard and eyebrows in a patient of KFSD |
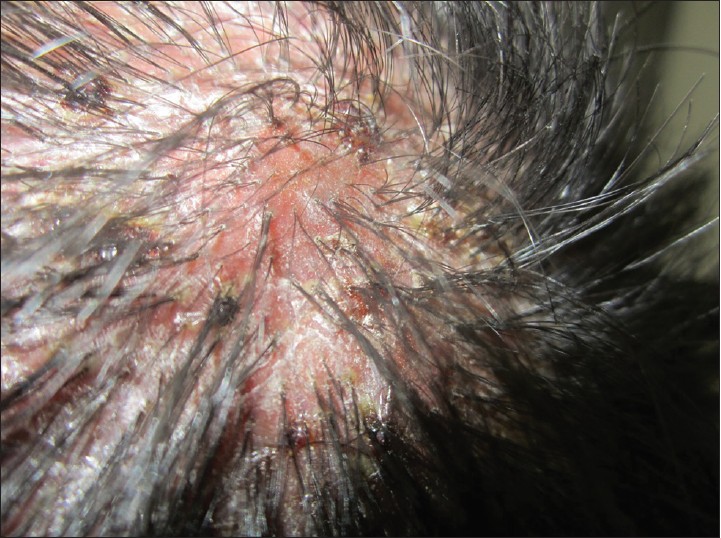 |
| Figure 10: Folliculitis Decalvans showing scarred scalp with yellowish, crusted pustules at the periphery with matted and tufted hairs in the centre of plaque |
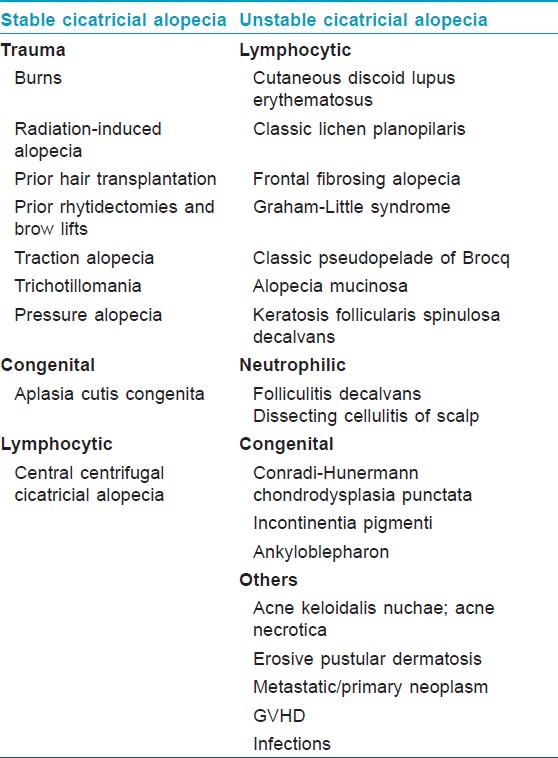
Another classification proposed to facilitate determination of the most suitable surgical corrective therapies for CA, categorize it into "stable" and "unstable" type [Table - 2]. [8] "Stable" CAs are secondary to isolated events that cause permanent scarring in a hair-bearing region. Once corrected surgically, there is no need for constant vigilance. Whereas "unstable" cicatricial alopecias (UCAs) are secondary to disorders that have a tendency to progress and recur intermittently over the course of time. Therefore, prior to considering any surgical treatment, it is vital to identify the type of alopecia and also to confirm quiescence preferably for at least 1 year so that they can be successfully treated medically before scarring actually occurs. [8]
Pathogenesis
PCA is characterized by permanent destruction of hair follicles, progressive deposition of collagen, and is frequently associated with loss of sebaceous glands. The pathogenesis of PCA revolves mainly around the destruction of slow-cycling, pluripotent HFSCs. [9] These HFSCs are located in the ′bulge′ region of hair follicle in outer root sheath (i.e., at the site of attachment of the arrector pili muscle to the outer root sheath). These stem cells are thought to produce secondary germ cells or transient amplifying cells that migrate in a bidirectional fashion, undergoing coordinated differentiation to restore and renew the upper follicle, including the sebaceous gland, and adjacent epidermis; and regrow the lower hair follicle during normal telogen-anagen cycling. [10]
But, HFSCs destruction alone cannot explain the full phenotypic profile seen in different PCA entities like erythema, epidermal atrophy, follicular plugging. Therefore, other pathogenic processes may account for such changes. [11]
The newer insights into the pathogenesis of PCA mainly involves the HFSCs destruction theories, impairment of self maintenance of HFSCs, alteration of lipid metabolism, neurogenic inflammation theory, environment, and genetic factors. [4],[11],[12],[13],[14],[15],[16],[17],[18],[19],[20],[21],[22],[23],[24],[25],[26],[27],[28],[29],[30],[31],[32],[33],[34],[35],[36],[37],[38],[39],[40],[41],[42],[43],[44],[45],[46],[47],[48],[49],[50],[51],[52],[53],[54]
Hair follicle stem cells destruction theory
This theory is based upon the observation of the peri-follicular inflammatory infiltrate relative to the two key structures of the hair follicle, the hair bulb (where the hair shaft is generated), and the hair bulge. In the prototypic reversible autoimmune hair loss disorder alopecia areata (AA), the inflammatory infiltrate is centered around the hair bulb; whereas in PCA, the infiltrate is mainly located around the bulge region and distal (noncycling) follicle. This marked difference in the intradermal location of the inflammatory infiltrate has major clinical consequences, that is, reversible versus permanent hair loss. [11],[12],[13]
Further, immuno-staining of keratin 15, (a recognized hair ′bulge′ marker in humans) has shown to be diminished or absent from the bulge region in LPP and CCLE with predominantly dense peri-follicular inflammatory infiltrates, suggesting that the inflammatory process is destroying the HFSCs pool. [14],[15]
Similarly, other studies showed that the bulge region, marked by the biomarkers such as keratin-15 and -19 and CD200, is preferentially affected and replaced by fibrotic tissue in PCA leading to permanent hair loss. [16],[17] Recent studies reported some cell populations marked by MTS24, [18] Lrig1, [19] Nestin, [20] Lgr5, [21] and Lgr6 [22] are also capable of reconstituting hair follicles, suggesting that these cells are endowed with some stem cell characteristics. But the inflammation and subsequent fibrosis in PCA affect most of these cell populations also, except Lgr5 expressing cells. [4]
Collapse of hair follicle immune privilege
Hair follicles provide major portal for entry of microbial agents but in immuno-competent individuals the clinical consequences of such invasions are rare, this is because of anti-infection defense mechanisms like intraepithelial T-cells, Langerhans cells, perifollicular mast cells, macrophages, and antimicrobial peptides (human β2-defensin, psoriasin, cathelicidin, and RNAse7). These substances together with various chemokines and proinflammatory molecules can cause massive inflammation, which can also breach the follicular epithelium. But the hair follicle has established a state of immuno-suppression known as ′immune privilege′ in its bulb and bulge region. This is done by the expression of potent endogenous immuno-suppressants like TGF-β1 and 2 (secretes macrophage inhibitory factor), α-MSH in hair follicular epithelium and low or absent expression of MHC class I and II molecules around the bulb and bulge. [11]
′Hair follicle immune privilege collapse′ is thus an attractive theory to explain the exposure of HFSCs to immune-mediated attack in PCA. The data supporting this show the immune reactivity of MHC class I, β2-microblobulin, and MHC class II, is upregulated in the hair ′bulge′ region of lesional skin, compared with uninvolved skin, in different PCA entities. [23]
Cytotoxic cell mediated hair follicle damage and proinflammatory response leading to PCA
This hypothesis is based upon the inflammatory infiltrate observed in the scalp biopsies of CCLE, LPP, and FD. In scarred areas of CCLE, there is upregulation of γδ-T-lymphocytes with increased expression of chemokine 4, co-localization of skin homing marker cutaneous lymphocyte antigen (CLA) with the cytotoxic marker granzyme B leading to direct cytotoxic tissue damage. [4],[11] Various proinflammatory cytokines like INF-γ, TNF-α, IL-2 are upregulated in the lesional skin of LPP, CCLE, and FD, leading to direct tissue damage and hence scarring. [4],[11]
Increased apoptosis in PCA
Apoptotic keratinocytes are commonly observed during the histological assessment of PCA, suggesting a potential role of apoptosis in these disorders. In CCLE, apoptosis is increased in the hair follicle epidermis with the upregulation of epithelial proliferation marker Ki-67 and the tumor suppressor gene p53. The p53 direct the damaged cells into apoptosis. [24],[25] Also, the expression of the constitutive apoptosis inhibitor Bcl-2 is reduced in CCLE, further predisposing to apoptosis-related tissue injury. [23] Peri-follicular FASL+ infiltrate is thought to play a key role in the follicular destruction commonly seen in CCLE through inappropriate apoptosis induction. [24],[26]
Lipid metabolism dysregulation in PCA pathogenesis
With an insufficient evidence, this hypothesis is based upon the observation of spontaneous mutation, in widely studied mouse model for PCA. [27] It states that defect in stearoyl-CoA desaturase 1 (required for sebaceous gland fatty acid composition) results in sebaceous glands atrophy and abnormal gland secretions, which in turn lead to delayed inner root sheath disintegration, retrograde hair shaft growth and penetration of the bulb, with resulting foreign body reaction and eventual destruction of the hair follicle. [27],[28] This supports the idea that inflammation in scarring alopecia is a secondary event resulting from a primary defect in the pilosebaceous unit. [29]
Another evidence supporting this hypothesis, is a recent study on decreased expression of genes for peroxisome proliferator-activated receptor γ (PPARγ) (required for lipid metabolism) and peroxisome biogenesis which triggers the pathogenesis of LPP. [30] The study states that decrease in expression of these genes led to progressive loss of peroxisomes, accumulation of proinflammatory lipids, and infiltration of inflammatory cells around the pilosebaceous structures followed by destruction of the complete pilosebaceous unit. [30] Based upon this theory, the beneficial role of PPARγ agonist in LPP has been described. [31] (Detailed more under "Management" section.)
Impaired self-maintenance of hair follicle stem cells cause hair loss
An experimental study, reported the loss of interaction between Col17a1 and HFSCs (which impairs the self-renewal capacity of stem cells) resulting in permanent hair loss in mice. It also demonstrates the change in microenvironment, including decreased extracellular matrix expression, causing scarring alopecia. Thus, it is possible that impaired self-maintenance or loss of self-regenerative potential due to environmental changes may also be responsible for PCA development. [32]
Neurogenic theory
It explains the possible role of psycho-emotional stress in PCA. The substance P (SP) a neuropeptide molecule, which is increased in the state of psycho-emotional stress, is an inducer of mast-cell dependent neurogenic inflammation. This results in dense peri-follicular inflammatory cell infiltrates (especially around the bulge), accumulation and degranulation of peri-follicular mast cells, increased hair follicular keratinocyte apoptosis and reduced hair matrix keratinocyte proliferation, which is followed by premature entry of hair follicle into catagen. [33],[34],[35]
SP when added to the cultures of human hair follicles in anagen phase, upregulates the expression of MHC class I molecule and β2 microglobulin in peri-follicular region, leading to collapse of immune privilege and thus giving way to HFSCs destruction. [36]
SP is also a known fibroblast growth factor (FGF), thus promoting fibrosis and scar formation after inflammatory damage. [37]
Environmental factors contributing PCA
Various environmental triggering factors for PCA are proposed like infections, trauma, drugs, etc. The role of Staphylococcus aureus in FD is well known, the abnormal host immune responses to the bacterial antigen are responsible for the intense inflammation and scarring alopecia in the disease. [5],[6]
CCCA, [38] FD, [39] AK, [40] and erosive pustular dermatosis of scalp [5],[6] are associated with traumatic hair care practices.
Drug therapies are also implicated in PCAs. Graham-Little syndrome has been reported following hepatitis B vaccination, [41] AK has been associated with anticonvulsant and ciclosporin therapy, [42],[43] and drug-induced cases of AM, [44] CCLE [45] have been described. Similarly, occurrence of LPP is reported after ingestion of gold, [46] hepatitis B vaccination [41] and hepatitis C infection. [47]
Genetic factors related to PCA
Several reports of the familial PCA cases in PPB, FD, GLP syndrome, and KFSD suggested the existence of genetic factors. [48],[49],[50],[51] X-linked KFSD represent genetic scarring alopecia, which is caused by a missense mutation in the MBTSP2 gene. [51] Thus, genetic factors are likely to underline the pathogenesis of several PCA entities in some form, either by predisposing an individual to a certain disorder or through a direct genetic effect, causing the condition. Further genetic studies of the families affected are required to identify the genes associated.
Clinical Features
The characteristic clinical features of each type of PCA are enumerated in [Table - 1]. A new entity described is ′cicatricial marginal alopecia′ (CMA). [67] In this condition, the patient presents with an unusual clinical pattern of alopecia with severe loss of hairs from a wide band-like area on the margins of scalp. The site of involvement most commonly is occipital scalp (40% cases), other being temporal area. On close examination of scalp, there is no clinical appreciable scarring, atrophy, or erythema. Clinical differential diagnosis of CMA includes AA and tractional alopecia (late stages). But there is no history of hair care practices leading to tractional hair loss. Histologically, CMA reveals replacement of hair follicle by columns of fibrous tissue at or below the level of isthmus, with retention of sebaceous glands. This pattern of fibrosis was consistent with scarring alopecia. [67]
Diagnosis
Dermoscopy/Trichoscopy
Dermatoscope is a convenient instrument that aids in diagnosis of skin and scalp lesion. It works on the principal of light reflected by the specimens. This is done by both hand-held dermatoscope using polarized light as well as with video dermatoscopy, which ensures 1000 times magnification. [68],[69]
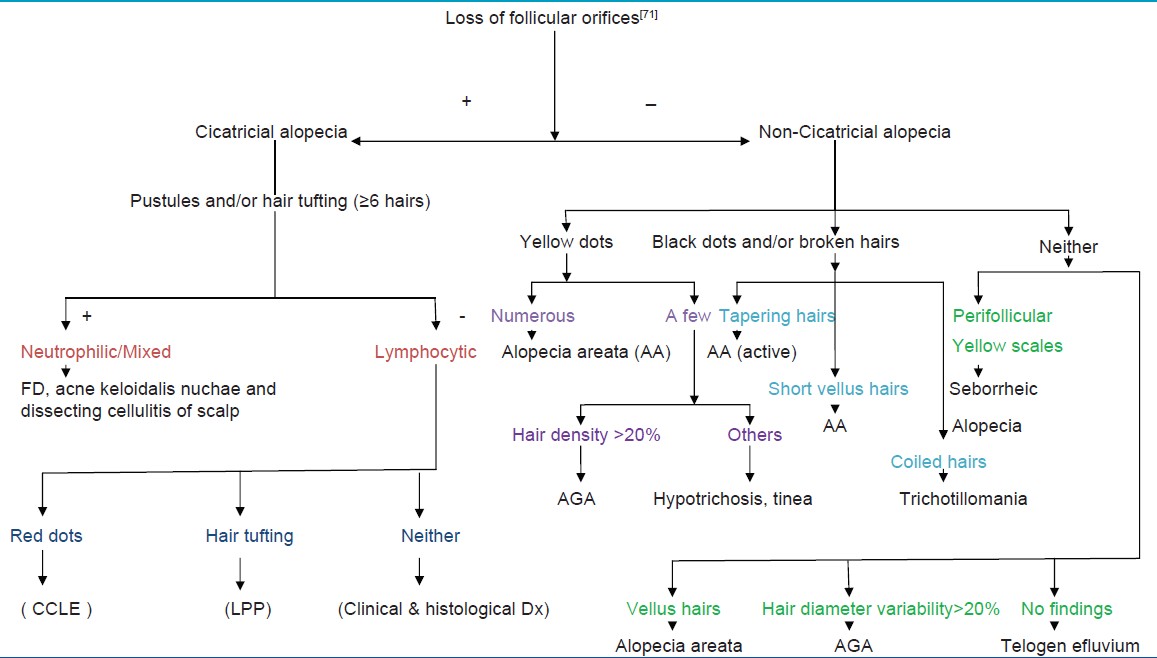
Dermoscopy of PCA reveals absence of follicular ostia in 100% cases even if it is not evident clinically, other findings suggestive of PCAs are tufted hairs, follicular hyperkeratosis, pili torti, and pink-white appearance. [68],[70],[71] Trichoscopy also helps clinicians assessing PCA disease activity, for example, "follicular red dots", erythematous polycyclic, concentric structures regularly distributed in and around the follicular ostia, are suggestive of active lupus erythematosus of the scalp. [70],[71] A recent review, gives an algorithmic description of trichoscopic features of CA and non-CA [Table - 3]. [71] This further emphasizes that trichoscopy provides the excellent first-line, noninvasive method for assessing scalp and hair characteristics in clinics giving important clues to diagnosis.
Reflectance confocal microscopy
Reflectance Confocal Microscopy (RCM) is an in vivo, noninvasive, repeatable technique of real-time, en-face microscopic imaging of the superficial layers of the skin down to the superficial reticular dermis, with resolution at cellular level close to conventional histopathology. [72],[73],[74],[75],[76],[77],[78]
RCM has been used for the evaluation of several inflammatory skin conditions, such as acute contact dermatitis, [73] psoriasis, [74] nonscarring alopecia, [75],[76] and in assessment of CCLE. [77]
A study evaluating RCM features of PCA (mainly LPP and CCLE) reveals epidermal disarray, spongiosis, exocytosis of inflammatory cells in the epidermis, interface dermatitis, peri- and intraadnexal infiltration of inflammatory cells, dilated vessels in the dermis, dermal infiltration of inflammatory cells, melanophages, and dermal sclerosis as features of PCA. It also differentiated LPP and CCLE based upon RCM features and has shown strong reduction to disappearance of inflammatory cells with treatment. [78]
Thus, RCM is a high-resolution imaging technique that may be helpful in the diagnosis and follow-up of scarring alopecia. It may also help in choosing the most appropriate biopsy site for more informative histology.
Histopathology
Histopathology of scalp is an essential tool in distinguishing CA from non-CA and in diagnosing the different PCA depending upon the inflammatory infiltrate. For an accurate histopathological diagnosis of PCA, multiple biopsy samples are obtained from active sites and carefully sectioned both vertically and transversely. [2] Unlike in other skin diseases, the information obtained by vertical sections is limited in hair disorders. Transverse sections enable both qualitative (e.g., inflammatory change, fibrosis) and quantitative (e.g., hair follicle numbers, size, phase of hair cycle) examination of scalp biopsy samples. [79]
Recently, the "HoVert" technique, a novel processing technique that produces transverse (horizontal) and vertical sections from a single biopsy has been described. This overcomes the limitation of multiple scalp biopsies [Figure - 11]. [80]
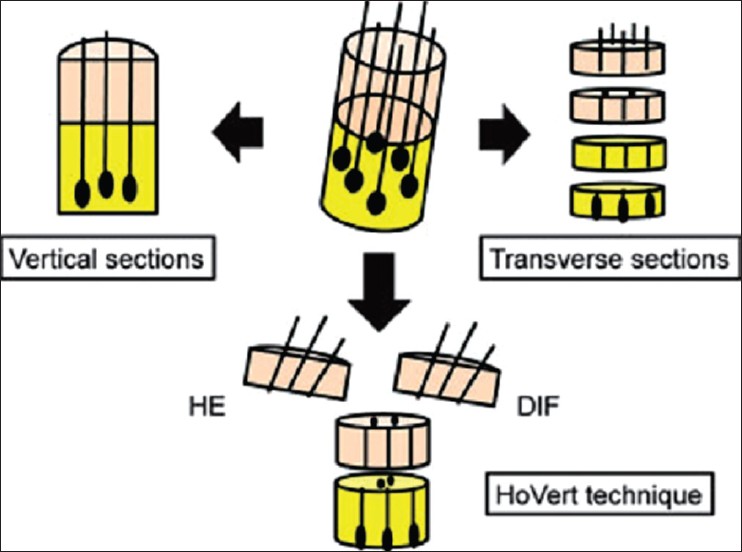 |
| Figure 11: 'Hovert' technique |
Based upon the histopathological picture, the CAs are divided mainly into lymphocyte-mediated primary cicatricial alopecia (LMPCA), neutophil-mediated primary cicatricial alopecia (NMPCA), and mixed CA [Table - 1]. [7],[81],[82] The diseases clubbed under LMPCA (LPP, FFA, DLE, CCCA, PPB) are typified by a lymphocyte rich infiltrate in early stages and with variable but limited fibrosis concentrated in the perifollicular adventitial unit in later stages. In contrast, in NMPCA group (FD, TF), there is a neutrophil-rich infiltrate in early disease, a mixed infiltrate in later disease, and marked scarring that extends beyond the peri-follicular dermis and extensively involves the reticular (inter-follicular) dermis. [103],[104] In mixed CA group entities like AKN, AN, EPD inflammatory infiltrate is mixed lympho-plasmacytic or neutrophilic. [7]
The differentiation between the LMPCA and NMPCA group is easier in early stages of inflammation, whereas in later stages the differences in all PCAs as well as late stage of non-CA are subtle. To solve this query one study suggested, that the presence of plasma cells within the infiltrate in a given biopsy serve as a clue to a diagnosis of NMPCA, [83] whereas another points that the presence of ′compound follicle′ (complex follicular unit fused at the level of the infundibulum) are a clue to diagnosis CA. [84] In yet another study, the number of ′compound follicles′ was suggested to distinguish between LMPCA and NMPCA with fewer (two or three) in the former and more melded follicles (four, five or more) in the latter. [85] In a recent study, ′eyes′ or ′goggles′ patterns of fibrosis observed at lower power at the level of infundibulum has been considered to be suggestive of LMPCA. [86]
Direct immunofluorescence study
It is an important diagnostic tool differentiating between PCA due to LPP or CCLE. In CCLE, it shows granular deposits of immunoglobulin (IgG) and complement (C3) at the dermoepidermal junction, while in LPP there are globular deposits of IgM adjacent to the hair follicles or at the dermoepidermal junction [Table - 1]. [52],[53],[54],[55],[56],[87]
Microarray analysis
Microarray analysis represents the global gene expression profile, as a diagnostic tool for clinically or pathologically indistinguishable PCA. Recently, a report of microarray analysis generated from total RNA isolates from active lesions of LPP and PPB, distinguished the two conditions, which were thought to be related. [88] However, further studies in this field may elucidates the genetic and molecular markers of various PCAs.
Management
PCA provides both the diagnostic and therapeutic dilemma to the treating dermatologist. The aim of treatment currently focuses the reduction of symptoms and to reduce or stop the progression of the disease. A general rule followed is to treat LMPCA with immunosuppression and NMPCA with antimicrobials or dapsone. [60] The step ladder treatment of PCA is given in [Table - 4].

Newer in treatment of LPP is the use of PPARγ agonists based upon the recent theory in pathogenesis suggesting the role of abnormal functioning PPARγ receptor as an initial trigger of inflammation in LPP. [30],[31] In a case report, pioglitazone Hcl (PPARγ agonist) 15 mg once daily was given to a patient of unstable LPP, in whom various treatment modalities including oral corticosteriods, antimalarials, MMF, failed to control the disease activity. With 8 months of pioglitazone, there were reportedly decrease in signs and symptoms along with reduction in inflammatory infiltrate in scalp biopsy. [31]
Thiazolidinediones or glitazones act by increasing activity of the nuclear receptor PPARγ and have antiinflammatory, antiproliferative, immunomodulatory effects, which includes downregulation of proinflammatory nuclear transcription factors, proteolytic enzymes, and interleukins. Thus, it helps in lipid biogenesis in pilosebaceous units and is antiinflammatory, immunomodulatory in active stage of LPP. [176],[177]
Surgical treatment of scarring alopecia includes hair transplantation, scalp reduction or alopecia reduction surgeries, tissue expansion, and flap surgeries. [8],[178],[179] Decision for surgical treatment is based upon the stability of the CA, because best results can only be obtained in stable cicatricial alopecia cases. [8] Depending upon the duration of stable disease, some authors recommend the surgical therapies after at least 1 year of quiescence, [8] whereas others state that this should be after 2 years of disease-free interval. [60] The other factors that require consideration before planning the surgical treatment for CA includes vigilance regarding possible evolution of future androgenetic alopecia in the patient under treatment, availability of donor hairs and donor-recipient area ratio, vascular supply to the recipient area, scalp laxity, patient′s healing characteristics, and location of the subsequent scars. [8]
The surgical treatment is individualized depending upon the above mentioned factors but in general surgical excision or alopecia reduction surgeries are preferred for both unstable and stable CA. The success of hair transplantation in CA is limited by various reasons like disproportionate donor-recipient area ratio, decreased survival of hair grafts in scarred area due to compromised vascular supply and relapses of the disease process leading to new areas of scarring alopecia. However, in view of recent platelet rich plasma therapies to provide growth factors for grafted hairs and to increase graft survival at recipient site, hair transplantation will provide better results in stable CA.
Conclusion
PCA is ′trichology emergency′ situation, in which lack of prompt and early treatment will lead to the inevitable loss of hair follicles along with permanent scarring. Newer pathogenesis has given the platform for development of emerging treatment modalities. But still a great deal of research is required in this field, may be developing viable stem cell therapies or bioengineered human hair follicles are the answers to it in future.
| 1. |
Whiting DA. Cicatricial alopecia: Clinico-pathological findings and treatment. Clin Dermatol 2001;19:211-5.
[Google Scholar]
|
| 2. |
Tan E, Martinka M, Ball N, Shapiro J. Primary cicatricial alopecias: Clinicopathology of 112 cases. J Am Acad Dermatol 2004;50:25-32.
[Google Scholar]
|
| 3. |
Griffin LL, Michaelides C, Griffiths CE, Paus R, Harries MJ. Primary cicatricial alopecias: A UK survey. Br J Dermatol 2012;167:692-705.
[Google Scholar]
|
| 4. |
Ohyama M. Primary cicatricial alopecia: Recent advances in understanding and management. J Dermatol 2012;39:18-26.
[Google Scholar]
|
| 5. |
Paus R, Olsen E, Messenger A. Hair growth disorders. In: Wolff K, Goldsmith LA, Katz SI, Gilchrest BA, Paller AS, Leffell DJ, editors. Fitzpatrick's Dermatology in General Medicine. 7 th ed. New York: McGraw-Hill; 2008. p. 753.
th ed. New York: McGraw-Hill; 2008. p. 753.'>[Google Scholar]
|
| 6. |
Sinclair RD. Acquired cicatricial alopecia. In: Burns T, Breathnach SM, Cox N, Griffiths CE, editors. Rook's Textbook of Dermatology. 8 th ed. Oxford: Wiley-Blackwill Publishing; 2010. p. 66.38-66.52.
th ed. Oxford: Wiley-Blackwill Publishing; 2010. p. 66.38-66.52.'>[Google Scholar]
|
| 7. |
Olsen EA, Bergfeld WF, Cotsarelis G, Price VH, Shapiro J, Sinclair R, et al. Summary of North American Hair Research Society (NAHRS)-sponsored Workshop on Cicatricial Alopecia, Duke University Medical Center, February 10 and 11, 2001. J Am Acad Dermatol 2003;48:103-10.
[Google Scholar]
|
| 8. |
Unger W, Unger R, Wesley C. The surgical treatment of cicatricial alopecia. Dermatol Ther 2008;21:295-311.
[Google Scholar]
|
| 9. |
Sellheyer K, Bergfeld WF. Histopathologic evaluation of alopecias. Am J Dermatopathol 2006;28:236-59.
[Google Scholar]
|
| 10. |
Lavker RM, Sun TT, Oshima H, Barrandon Y, Akiyama M, Ferraris C, et al. Hair follicle stem cells. J Invest Dermatol 2003;8:28-38.
[Google Scholar]
|
| 11. |
Harries MJ, Paus R. The pathogenesis of primary cicatricial alopecias. Am J Pathol 2010;177:2152-62.
[Google Scholar]
|
| 12. |
Cotsarelis G. Epithelial stem cells: A folliculocentric view. J Invest Dermatol 2006;126:1459-68.
[Google Scholar]
|
| 13. |
Paus R, Cotsarelis G. The biology of hair follicles. N Engl J Med. 1999;341:491-7.
[Google Scholar]
|
| 14. |
Pozdnyakova O, Mahalingam M. Involvement of the bulge region in primary scarring alopecia. J Cutan Pathol 2008;35:922-5.
[Google Scholar]
|
| 15. |
Al-Refu K, Edward S, Ingham E, Goodfield M. Expression of hair follicle stem cells detected by cytokeratin 15 stain: Implications for pathogenesis of the scarring process in cutaneous lupus erythematosus. Br J Dermatol 2009;160:1188-96.
[Google Scholar]
|
| 16. |
Cotsarelis G, Millar SE. Towards a molecular understanding of hair loss and its treatment. Trends Mol Med 2001;7:293-301.
[Google Scholar]
|
| 17. |
McElwee KJ. Etiology of cicatricial alopecias: A basic science point of view. Dermatol Ther 2008;21:212-20.
[Google Scholar]
|
| 18. |
Nijhof JG, Braun KM, Giangreco A. The cell-surface marker MTS24 identifies a novel population of follicular keratinocytes with characteristics of progenitor cells. Development 2006;133:3027-37.
[Google Scholar]
|
| 19. |
Jensen KB, Collins CA, Nascimento E. Lrig1 expression defines a distinct multipotent stem cell population in mammalian epidermis. Cell Stem Cell 2009;4:427-39.
[Google Scholar]
|
| 20. |
Amoh Y, Li L, Katsuoka K, Penman S, Hoffman RM. Multipotent nestin-positive, keratin-negative hair-follicle bulge stem cells can form neurons. Proc Natl Acad Sci USA 2005;102:5530-4.
[Google Scholar]
|
| 21. |
Jaks V, Barker N, Kasper M, van Es JH, Snippert HJ, Clevers H, et al. Lgr5 marks cycling, yet long-lived, hair follicle stem cells. Nat Genet 2008;40:1291-9.
[Google Scholar]
|
| 22. |
Snippert HJ, Haegebarth A, Kasper M, Jaks V, van Es JH, Barker N, et al. Lgr6 marks stem cells in the hair follicle that generate all cell lineages of the skin. Science 2010;327:1385-9.
[Google Scholar]
|
| 23. |
Harries MJ, Meyer KC, Chaudhry IH, Griffiths CE, Paus R. Does collapse of immune privilege in the hair-follicle bulge play a role in the pathogenesis of primary cicatricial alopecia? Clin Exp Dermatol 2010;35:637-44.
[Google Scholar]
|
| 24. |
Baima B, Sticherling M. Apoptosis in different cutaneous manifestations of lupus erythematosus. Br J Dermatol 2001;144:958-66.
[Google Scholar]
|
| 25. |
Pablos JL, Santiago B, Galindo M, Carreira PE, Ballestin C, Gomez-Reino JJ. Keratinocyte apoptosis and p53 expression in cutaneous lupus and dermatomyositis. J Pathol 1999;188:63-8.
[Google Scholar]
|
| 26. |
Nakajima M, Nakajima A, Kayagaki N, Honda M, Yagita H, Okumura K. Expression of Fas ligand and its receptor in cutaneous lupus: implication in tissue injury. Clin Immunol Immunopathol 1997;83:223-9.
[Google Scholar]
|
| 27. |
Zheng Y, Eilertsen KJ, Ge L, Zhang L, Sundberg JP, Prouty SM, et al. Scd1 is expressed in sebaceous glands and is disrupted in the asebia mouse. Nat Genet 1999;23:268-70.
[Google Scholar]
|
| 28. |
Sundberg JP, Boggess D, Sundberg BA, Eilertsen K, Parimoo S, Filippi M, et al. Asebia-2J (Scd1(ab2J)): a new allele and a model for scarring alopecia. Am J Pathol 2000;156:2067-75.
[Google Scholar]
|
| 29. |
Stenn KS. Insights from the asebia mouse: A molecular sebaceous gland defect leading to cicatricial alopecia. J Cutan Pathol 2001;28:445-7.
[Google Scholar]
|
| 30. |
Karnik P, Tekeste Z, McCormick TS, Gilliam AC, Price VH, Cooper KD, et al. Hair follicle stem cell-specific PPARγ deletion causes scarring alopecia. J Invest Dermatol 2009;129:1243-57.
[Google Scholar]
|
| 31. |
Mirmirani P, Karnik P. Lichen planopilaris treated with a peroxisome proliferator-activated receptor γ agonist. Arch Dermatol 2009;145:1363-6.
[Google Scholar]
|
| 32. |
Tanimura S, Tadokoro Y, Inomata K, Binh NT, Nishie W, Yamazaki S, et al. Hair follicle stem cells provide a functional niche for melanocyte stem cells. Cell Stem Cell 2011;8:177-87.
[Google Scholar]
|
| 33. |
Peters EM, Arck PC, Paus R. Hair growth inhibition by psychoemotional stress: A mouse model for neural mechanisms in hair growth control. Exp Dermatol 2006;15:1-13.
[Google Scholar]
|
| 34. |
Peters EM, Botchkarev VA, Botchkareva NV, Tobin DJ, Paus R. Hair-cycle-associated remodeling of the peptidergic innervation of murine skin, and hair growth modulation by neuropeptides. J Invest Dermatol 2001;116:236-45.
[Google Scholar]
|
| 35. |
Arck PC, Handjiski B, Hagen E, Joachim R, Klapp BF, Paus R. Indications for a 'brain-hair follicle axis (BHA)': Inhibition of keratinocyte proliferation and up-regulation of keratinocyte apoptosis in telogen hair follicles by stress and substance P. FASEB J 2001;15:2536-8.
[Google Scholar]
|
| 36. |
Peters EM, Liotiri S, Bodo E, Hagen E, Biro T, Arck PC, et al. Probing the effects of stress mediators on the human hair follicle: Substance P holds central position. Am J Pathol 2007;171:1872-86.
[Google Scholar]
|
| 37. |
Nilsson J, von Euler AM, Dalsgaard CJ. Stimulation of connective tissue cell growth by substance P and substance K. Nature 1985;315:61-3.
[Google Scholar]
|
| 38. |
Sperling LC, Sau P. The follicular degeneration syndrome in black patients. 'Hot comb alopecia' revisited and revised. Arch Dermatol 1992;128:68-74.
[Google Scholar]
|
| 39. |
Salami T, Omeife H, Samuel S. Prevalence of acne keloidalis nuchae in Nigerians. Int J Dermatol 2007;46:482-4.
[Google Scholar]
|
| 40. |
Dalziel KL, Telfer NR, Wilson CL, Dawber RP. Tufted folliculitis. A specific bacterial disease? Am J Dermatopathol 1990;12:37-41.
[Google Scholar]
|
| 41. |
Bardazzi F, Landi C, Orlandi C, Neri I, Varotti C. Graham Little-Piccardi-Lasseur syndrome following HBV vaccination. Acta Derm Venereol 1999;79:93.
[Google Scholar]
|
| 42. |
Grunwald MH, Ben-Dor D, Livni E, Halevy S. Acne keloidalis-like lesions on the scalp associated with antiepileptic drugs. Int J Dermatol 1990;29:559-61.
[Google Scholar]
|
| 43. |
Carnero L, Silvestre JF, Guijarro J, Albares MP, Botella R. Nuchal acne keloidalis associated with cyclosporin. Br J Dermatol 2001;144:429-30.
[Google Scholar]
|
| 44. |
Magro CM, Crowson AN. Drug-induced immune dysregulation as a cause of atypical cutaneous lymphoid infiltrates: A hypothesis. Hum Pathol 1996;27:125-32.
[Google Scholar]
|
| 45. |
Vedove CD, Del Giglio M, Schena D, Girolomoni G. Drug-induced lupus erythematosus. Arch Dermatol Res 2009;301:99-105.
[Google Scholar]
|
| 46. |
Burrows NP, Grant JW, Crisp AJ, Roberts SO. Scarring alopecia following gold therapy. Acta Derm Venereol (Stockh) 1994;74:486.
[Google Scholar]
|
| 47. |
Brudy L, Janier M, Reboul D, Baviera E, Bonvalet D, Daniel F. Erosive lichen of scalp. Acta Derm Venereol 1997;124:703-6.
[Google Scholar]
|
| 48. |
Sahl WJ. Pseudopelade: An inherited alopecia. Int J Dermatol 1996;35:715-9.
[Google Scholar]
|
| 49. |
Douwes KE, Landthaler M, Szeimies RM. Simultaneous occurrence of folliculitis decalvans capillitii in identical twins. Br J Dermatol 2000;143:195-7.
[Google Scholar]
|
| 50. |
Viglizzo G, Verrini A, Rongioletti F. Familial Lassueur-Graham- Little-Piccardi syndrome. Dermatology 2004;208:142-4.
[Google Scholar]
|
| 51. |
Aten E, Brasz LC, Bornholdt D, Hooijkaas IB, Porteous ME, Sybert VP, et al. Keratosis follicularis spinulosa decalvans is caused by mutations in MBTPS2. Hum Mutat 2010;31:1125-33.
[Google Scholar]
|
| 52. |
Moises-Alfaro C, Berron-Perez R, Carrasco-Daza D, Gutierrez-Castrellon P, Ruiz Maldonado R. Discoid lupus erythematosus in children: clinical, histopathologic, and follow-up features in 27 cases. Pediatr Dermatol 2003;20:103-7.
[Google Scholar]
|
| 53. |
Ross EK, Tan E, Shapiro J. Update on primary cicatricial alopecias. J Am Acad Dermatol 2005;53:1-37.
[Google Scholar]
|
| 54. |
Sullivan JR, Kossard S. Acquired scalp alopecia. Part I: A review. Australas J Dermatol 1998;39:207-19.
[Google Scholar]
|
| 55. |
Chieregato C, Zini A, Barba A, Magnanini M, Rosina P. Lichen planopilaris: Report of 30 cases and review of the literature. Int J Dermatol 2003;42:342-5.
[Google Scholar]
|
| 56. |
Annessi G, Lombardo G, Gobello T, Puddu P. A clinicopathologic study of scarring alopecia due to lichen planus: Comparison with scarring alopecia in discoid lupus erythematosus and pseudopelade. Am J Dermatopathol 1999;21:324-31.
[Google Scholar]
|
| 57. |
Dawn G, Holmes S, Moffatt D, Munro C. Post-menopausal frontal fibrosing alopecia. Clin Exp Dermatol 2003;28:43-5.
[Google Scholar]
|
| 58. |
Bianchi L, Paro Vidolin A, Piemonte P, Carboni I, Chimenti S. Graham Little-Piccardi-Lassueur syndrome: Effective treatment with cyclosporin A. Clin Exp Dermatol 2001;26:518-20.
[Google Scholar]
|
| 59. |
Amato L, Massi D, Berti S, Moretti S, Fabbri P. A multiparametric approach is essential to define different clinicopathological entities within pseudopelade of Brocq: Reply from authors. Br J Dermatol 2002;146:532-3.
[Google Scholar]
|
| 60. |
Harries MJ, Sinclair RD, Donald-Hull M, Whiting DA, Griffiths CE, Paus R. Management of primary cicatricial alopecias: Options for treatment. Br J Dermatol 2008;159:1-22.
[Google Scholar]
|
| 61. |
Cerroni L, Fink-Puches R, Back B, Kerl H. Follicular mucinosis: A critical reappraisal of clinicopathologic features and association with mycosis fungoides and Se'zary syndrome. Arch Dermatol 2002;138:182-9.
[Google Scholar]
|
| 62. |
Powell JJ, Dawber RP. Successful treatment regime for folliculitis decalvans despite uncertainty of all aetiological factors. Br J Dermatol 2001;144:428-9.
[Google Scholar]
|
| 63. |
Brooke RC, Griffiths CE. Folliculitis decalvans. Clin Exp Dermatol 2001;26:120-2.
[Google Scholar]
|
| 64. |
Ljubojevic S, Pasic A, Lipozencic J, Skerlev M. Perifolliculitis capitis abscedens et suffodiens. J Eur Acad Dermatol Venereol 2005;19:719-21.
[Google Scholar]
|
| 65. |
Sperling LC, Homoky C, Pratt L, Sau P. Acne keloidalis is a form of primary scarring alopecia. Arch Dermatol 2000;136:479-84.
[Google Scholar]
|
| 66. |
Van exel CE, English JC 3 rd . Erosive pustular dermatosis of the scalp and nonscalp. J Am Acad Dermatol 2007;57:S11-4.
[Google Scholar]
|
| 67. |
Goldberg LJ. Cicatricial marginal alopecia: Is it all traction? Br J Dermatol 2009;160:62-8.
[Google Scholar]
|
| 68. |
Kose OK, Gulec T. Clinical evaluation of alopecias using a handheld dermatoscope. J Am Acad Dermatol 2012;67:206-14.
[Google Scholar]
|
| 69. |
Micali G, Lacarrubba F. Possible applications of videodermatoscopy beyond pigmented lesions. Int J Dermatol 2003;42:430-3.
[Google Scholar]
|
| 70. |
Tosti A, Torres F, Misciali C, Vincenzi C, Starace M, Miteva M, et al. Follicular red dots: A novel dermoscopic pattern observed in scalp discoid lupus erythematosus. Arch Dermatol 2009;145:1406-9.
[Google Scholar]
|
| 71. |
Inui S. Trichoscopy: A new frontier for the diagnosis of hair diseases. Expert Rev Dermatol 2012;7:1-8.
[Google Scholar]
|
| 72. |
Marghoob AA, Halpern A. Confocal scanning laser reflectance microscopy. Arch Dermatol 2005;141:212-5.
[Google Scholar]
|
| 73. |
Astner S, Gonzalez E, Cheung A, Rius-Diaz F, González S. Pilot study on the sensitivity and specificity of in vivo reflectance confocal microscopy in the diagnosis of allergic contact dermatitis. J Am Acad Dermatol 2005;53:986-92.
[Google Scholar]
|
| 74. |
Ardigo` M, Cota C, Berardesca V, Gonza'lez S. Concordance between in vivo reflectance confocal microscopy and histology in the evaluation of plaque psoriasis. J Am Acad Dermatol 2009;23:660-7.
in vivo reflectance confocal microscopy and histology in the evaluation of plaque psoriasis. J Am Acad Dermatol 2009;23:660-7.'>[Google Scholar]
|
| 75. |
Ardigò M, Tosti A, Cameli N, Vincenzi C, Misciali C, Berardesca E. Reflectance confocal microscopy of the yellow dot pattern in alopecia areata. Arch Dermatol 2011;147:61-4.
[Google Scholar]
|
| 76. |
Rudnicka L, Olszewska M, Rakowska A. In vivo reflectance confocal microscopy: Usefulness for diagnosing hair diseases. J Dermatol Case Rep 2008;4:55-9.
[Google Scholar]
|
| 77. |
Ardigò M, Maliszewski I, Cota C, Scope A, Sacerdoti G, Gonzalez S, et al. Preliminary evaluation of in vivo reflectance confocal microscopy features of discoid lupus erythematosus. Br J Dermatol 2007;156:1196-203.
[Google Scholar]
|
| 78. |
Agozzino M, Tosti A, Barbieri L, Moscarella E, Cota C, Berardesca E et al. Confocal microscopic features of scarring alopecia: Preliminary report. Br J Dermatol 2011;165:534-40.
[Google Scholar]
|
| 79. |
Sperling LC. Scarring alopecia and the dermatopathologist. J Cutan Pathol 2001;28:333-42.
[Google Scholar]
|
| 80. |
Nguyen JV, Hudacek K, Whitten JA, Rubin AI, Seykora JT. The HoVert technique: A novel method for the sectioning of alopecia biopsies. J Cutan Pathol 2011;38:401-6.
[Google Scholar]
|
| 81. |
Mirmirani P, Willey A, Headington JT, Stenn K, McCalmont TH, Price VH. Primary cicatricial alopecia: histopathologic findings do not distinguish clinical variants. J Am Acad Dermatol 2005;52:637-43.
[Google Scholar]
|
| 82. |
Stefanato CM. Histopathology of alopecia: A clinicopathological approach to diagnosis. Histopathology 2010;56:24-7.
[Google Scholar]
|
| 83. |
Sullivan JR, Kossard S. Aquired scalp alopecia. Part II: A Review. Australas J Dermatol 1999;40:61-70.
[Google Scholar]
|
| 84. |
Powell JJ, Dawber RP, Gatter K. Folliculitis decalvans including tufted folliculitis: Clinical, histological and therapeutic findings. Br J Dermatol 1999;140:328-33.
[Google Scholar]
|
| 85. |
Pincus LB, Price VH, McCalmont TH. The amount counts: Distinguishing neutrophil-mediated and lymphocyte-mediated cicatricial alopecia by compound follicles. J Cutan Pathol 2011;38:1-4.
[Google Scholar]
|
| 86. |
Miteva M, Torres F, Tosti A. The 'eyes' or 'goggles' as a clue to the histopathological diagnosis of primary lymphocytic cicatricial alopecia. Br J Dermatol 2012;166:454-5.
[Google Scholar]
|
| 87. |
Trachsler S, Trueb RM. Value of direct immunofluorescence for differential diagnosis of cicatricial alopecia. Dermatology 2005;211:98-102.
[Google Scholar]
|
| 88. |
Yu M, Bell RH, Ross EK, Lo BK, Isaac-Renton M, Martinka M, et al. Lichen planopilaris and pseudopelade of Brocq involve distinct disease associated gene expression patterns by microarray. J Dermatol Sci 2010;57:27-36.
[Google Scholar]
|
| 89. |
Werth V. Current treatment of cutaneous lupus erythematosus. Dermatol Online J 2001;7:2.
[Google Scholar]
|
| 90. |
McCauliffe DP. Cutaneous lupus erythematosus. Semin Cutan Med Surg 2001;20:14-26.
[Google Scholar]
|
| 91. |
Jewell ML, McCauliffe DP. Patients with cutaneous lupus erythematosus who smoke are less responsive to antimalarial treatment. J Am Acad Dermatol 2000;42:983-7.
[Google Scholar]
|
| 92. |
Chieregato C, Barba A, Zini A, Peroni A Jr, Magnanini M, Rosina P. Discoid lupus erythematosus: Clinical and pathological study of 24 patients. J Eur Acad Dermatol Venereol 2004;18:113-8.
[Google Scholar]
|
| 93. |
Al-Mutairi N, Rijhwani M, Nour-Eldin O. Hypertrophic lupus erythematosus treated successfully with acitretin as monotherapy. J Dermatol 2005;32:482-6.
[Google Scholar]
|
| 94. |
Coelho A, Souto MI, Cardoso CR, Salgado DR, Schmal TR, Waddington CM, et al. Long-term thalidomide use in refractory cutaneous lesions of lupus erythematosus: A 65 series of Brazilian patients. Lupus 2005;14:434-9.
[Google Scholar]
|
| 95. |
Housman TS, Jorizzo JL, McCarty MA, Grummer SE, Fleischer AB Jr, Sutej PG. Low-dose thalidomide therapy for refractory cutaneous lesions of lupus erythematosus. Arch Dermatol 2003;139:50-4.
[Google Scholar]
|
| 96. |
Tzung TY, Liu YS, Chang HW. Tacrolimus vs. clobetasol propionate in the treatment of facial cutaneous lupus erythematosus: A randomized, double-blind, bilateral comparison study. Br J Dermatol 2007;156:191-2.
[Google Scholar]
|
| 97. |
Heffernan MP, Nelson MM, Smith DI, Chung JH. 0.1% tacrolimus ointment in the treatment of discoid lupus erythematosus. Arch Dermatol 2005;141:1170-1.
[Google Scholar]
|
| 98. |
Sugano M, Shintani Y, Kobayashi K, Sakakibara N, Isomura I, Morita A. Successful treatment with topical tacrolimus in four cases of discoid lupus erythematosus. J Dermatol 2006;33:887-91.
[Google Scholar]
|
| 99. |
Tlacuilo-Parra A, Guevara-Gutierrez E, Gutierrez-Murillo F, Sorto-Ortiz A, Barba-Gomez F, Hernandez-Torres M, et al. Pimecrolimus 1% cream for the treatment of discoid lupus erythematosus. Rheumatology (Oxford) 2005;44:1564-8.
[Google Scholar]
|
| 100. |
Kreuter A, Gambichler T, Breuckmann F, Pawlak FM, Stucker M, Bader A, et al. Pimecrolimus 1% cream for cutaneous lupus erythematosus. J Am Acad Dermatol 2004;51:407-10.
[Google Scholar]
|
| 101. |
Ell JA, Burge S, Wojnarowska F. Vitamin E and discoid lupus erythematosus. Lupus 1992;1:303-5.
[Google Scholar]
|
| 102. |
Steinkjer B. Auranofin in the treatment of discoid lupus erythematosus. J Dermatol Treat 1991;2:27-9.
[Google Scholar]
|
| 103. |
Neri R, Mosca M, Bernacchi E, Bombardieri S. A case of SLE with acute, subacute and chronic cutaneous lesions successfully treated with dapsone. Lupus 1999;8:240-3.
[Google Scholar]
|
| 104. |
Hanjani NM, Nousari CH. Mycophenolate mofetil for the treatment of cutaneous lupus erythematosus with smoldering systemic involvement. Arch Dermatol 2002;138:1616-8.
[Google Scholar]
|
| 105. |
Wenzel J, Brahler S, Bauer R, Bieber T, Tuting T. Efficacy and safety of methotrexate in recalcitrant cutaneous lupus erythematosus: Results of a retrospective study in 43 patients. Br J Dermatol 2005;153:157-62.
[Google Scholar]
|
| 106. |
Huber A, Tuting T, Bauer R, Bieber T, Wenzel J. Methotrexate treatment in cutaneous lupus erythematosus: Subcutaneous application is as effective as intravenous administration. Br J Dermatol 2006;155:861-2.
[Google Scholar]
|
| 107. |
Martinez J, de Misa RF, Torrelo A. Low-dose intralesional interferon alfa for discoid lupus erythematosus. J Am Acad Dermatol 1992;26:494-6.
[Google Scholar]
|
| 108. |
Thivolet J, Nicolas JF, Kanitakis J, Lyonnet S, Chouvet B. Recombinant interferon alpha 2a is effective in the treatment of discoid and subacute cutaneous lupus erythematosus. Br J Dermatol 1990;122:405-9.
[Google Scholar]
|
| 109. |
Prinz JC, Meurer M, Reiter C. Treatment of severe cutaneous lupus erythematosus with a chimeric CD4 monoclonal antibody, cM-T412. J Am Acad Dermatol 1996;34:244-52.
[Google Scholar]
|
| 110. |
Vena GA, Coviello C, Mastrolonardo M. Topical 5-fluorouracil in the treatment of discoid lupus erythematosus. Preliminary study over two years. J Dermatol Treat 1996;7:167-9.
[Google Scholar]
|
| 111. |
Edwards KR, Burke WA. Treatment of localized discoid lupus erythematosus with tazarotene. J Am Acad Dermatol 1999;41:1049-50.
[Google Scholar]
|
| 112. |
Gerdsen R, Wenzel J, Uerlich M. Successful treatment of chronic discoid lupus erythematosus of the scalp with imiquimod. Dermatology 2002;205:416-8.
[Google Scholar]
|
| 113. |
Chieregato C, Zini A, Barba A. Lichen planopilaris: Report of 30 cases and review of the literature. Int J Dermatol 2003;42:342-5.
[Google Scholar]
|
| 114. |
Cevasco NC, Bergfeld WF, Remzi BK, Deknott HR. A case-series of 29 patients with lichen planopilaris: The Cleveland Clinic Foundation experience on evaluation, diagnosis, and treatment. J Am Acad Dermatol 2007;57:47-53.
[Google Scholar]
|
| 115. |
Reygagne P, Assouly P, Matard B. Oral ciclosporin in lichen planopilaris. Dermatology 2006;213:59.
[Google Scholar]
|
| 116. |
Mirmirani P, Willey A, Price VH. Short course of oral cyclosporine in lichen planopilaris. J Am Acad Dermatol 2003;49:667-71.
[Google Scholar]
|
| 117. |
Scalvenzi M, De Natale F, Forgione P. Topical cyclosporin A in the treatment of lichen planopilaris. Ann Ital Dermatol Clin Sper 1998;52:37-40.
[Google Scholar]
|
| 118. |
Tursen U, Api H, Kaya T. Treatment of lichen planopilaris with mycophenolate mofetil. Dermatol Online J 2004;10:24.
[Google Scholar]
|
| 119. |
George SJ, Hsu S. Lichen planopilaris treated with thalidomide. J Am Acad Dermatol 2001;45:965-6.
[Google Scholar]
|
| 120. |
Boyd AS, King LE Jr. Thalidomide-induced remission of lichen planopilaris. J Am Acad Dermatol 2002;47:967-8.
[Google Scholar]
|
| 121. |
Sehgal VN, Bajaj P, Srivastva G. Lichen planopilaris [cicatricial (scarring) alopecia] in a child. Int J Dermatol 2001;40:461-3.
[Google Scholar]
|
| 122. |
Stefanidou MP, Ioannidou DJ, Panayiotides JG. Low molecular weight heparin: A novel alternative therapeutic approach for lichen planus. Br J Dermatol 1999;141:1040-5.
[Google Scholar]
|
| 123. |
Vavricka BP, Haug S, Eliades I. 308-nm excimer laser treatment of lichen planopilaris of the scalp. Dermatology 2006;213:74.
[Google Scholar]
|
| 124. |
Moreno-Ramirez D, Camacho Martinez F. Frontal fibrosing alopecia: A survey in 16 patients. J Eur Acad Dermatol Venereol 2005;19:700-5.
[Google Scholar]
|
| 125. |
Tosti A, Piraccini BM, Iorizzo M. Frontal fibrosing alopecia in postmenopausal women. J Am Acad Dermatol 2005;52:55-60.
[Google Scholar]
|
| 126. |
Naz E, Vidaurrazaga C, Hernandez-Cano N. Postmenopausal frontal fibrosing alopecia. Clin Exp Dermatol 2003;28:25-7.
[Google Scholar]
|
| 127. |
Faulkner CF, Wilson NJ, Jones SK. Frontal fibrosing alopecia associated with cutaneous lichen planus in a premenopausal woman. Australas J Dermatol 2002;43:65-7.
[Google Scholar]
|
| 128. |
Vaisse V, Matard B, Assouly P, Jouannique C, Reygagne P. Postmenopausal frontal fibrosing alopecia: 20 cases. Ann Dermatol Venereol 2003;130:607-10.
[Google Scholar]
|
| 129. |
Ghislain PD, van Eeckhout P, Ghislain E. Lassueur-Graham Little-Piccardi syndrome: A 20-year follow-up. Dermatology 2003;206:391-2.
[Google Scholar]
|
| 130. |
Bianchi L, Paro Vidolin A, Piemonte P. Graham Little-Piccardi-Lassueur syndrome: Effective treatment with cyclosporin A. Clin Exp Dermatol 2001;26:518-20.
[Google Scholar]
|
| 131. |
Srivastava M, Mikkilineni R, Konstadt J. Lassueur-Graham Little-Piccardi syndrome. Dermatol Online J 2007;13:12.
[Google Scholar]
|
| 132. |
Viglizzo G, Verrini A, Rongioletti F. Familial Lassueur-Graham Little-Piccardi syndrome. Dermatology 2004;208:142-4.
[Google Scholar]
|
| 133. |
Anderson BE, Mackley CL, Helm KF. Alopecia mucinosa: Report of a case and review. J Cutan Med Surg 2003;7:124-8.
[Google Scholar]
|
| 134. |
Yotsumoto S, Uchimiya H, Kanzaki T. A case of follicular mucinosis treated successfully with minocycline. Br J Dermatol 2000;142:841-2.
[Google Scholar]
|
| 135. |
LoPresti P, Sammarco E, Baldo A. Follicular mucinosis: Treatment with topical indomethacin. Ann Ital Dermatol Clin Sper 1998;52:88-90.
[Google Scholar]
|
| 136. |
Rustin MH, Bunker CB, Levene GM. Follicular mucinosis presenting as acute dermatitis and response to dapsone. Clin Exp Dermatol 1989;14:382-4.
[Google Scholar]
|
| 137. |
Harthi FA, Kudwah A, Ajlan A. Urticaria-like follicular mucinosis responding to dapsone. Acta Derm Venereol (Stockh) 2003;83:389-90.
[Google Scholar]
|
| 138. |
Passaro EM, Silveira MT, Valente NY. Acneiform follicular mucinosis. Clin Exp Dermatol 2004;29:396-8.
[Google Scholar]
|
| 139. |
Arca E, Kose O, Tastan HB. Follicular mucinosis responding to isotretinoin treatment. J Dermatolog Treat 2004;15:391-5.
[Google Scholar]
|
| 140. |
Guerriero C, De Simone C, Guidi B. Follicular mucinosis successfully treated with isotretinoin. Eur J Dermatol 1999;9:22-4.
[Google Scholar]
|
| 141. |
von Kobyletzki G, Kreuter JA, Nordmeier R. Treatment of idiopathic mucinosis follicularis with UVA1 cold light phototherapy. Dermatology 2000;201:76-7.
[Google Scholar]
|
| 142. |
Meissner K, Weyer U, Kowalzick L. Successful treatment of primary progressive follicular mucinosis with interferons. J Am Acad Dermatol 1991;24:848-50.
[Google Scholar]
|
| 143. |
Baden HP, Byers HR. Clinical findings, cutaneous pathology, and response to therapy in 21 patients with keratosis pilaris atrophicans. Arch Dermatol 1994;130:469-75.
[Google Scholar]
|
| 144. |
Romine KA, Rothschild JG, Hansen RC. Cicatricial alopecia and keratosis pilaris. Keratosis follicularis spinulosa decalvans. Arch Dermatol 1997;133:381.
[Google Scholar]
|
| 145. |
Kunte C, Loeser C, Wolff H. Folliculitis spinulosa decalvans: Successful therapy with dapsone. J Am Acad Dermatol 1998;39:891-3.
[Google Scholar]
|
| 146. |
Puppin D, Aractingi S, Dubertret L. Keratosis follicularis spinulosa decalvans: Report of a case with ultrastructural study and unsuccessful trial of retinoids. Dermatology 1992;184:133-6.
[Google Scholar]
|
| 147. |
Chui CT, Berger TG, Price VH. Recalcitrant scarring follicular disorders treated by laser-assisted hair removal: A preliminary report. Dermatol Surg 1999;25:34-7.
[Google Scholar]
|
| 148. |
Fernandes JC, Correia TM, Azevedo F. Tufted hair folliculitis after scalp injury. Cutis 2001;67:243-5.
[Google Scholar]
|
| 149. |
Iwahara K, Ishii K, Chen Y. Tufted hair folliculitis: Response to topical therapy with nadifloxacin. Eur J Dermatol 1999;9:276-7.
[Google Scholar]
|
| 150. |
Pranteda G, Grimaldi M, Palese E. Tufted hair folliculitis: Complete enduring response after treatment with rifampicin. J Dermatolog Treat 2004;15:396-8.
[Google Scholar]
|
| 151. |
Stockmeier M, Kunte C, Feldmann K. Folliculitis decalvans -treatment with systemic rifampicin-clindamycin in 17 patients. Aktuel Dermatol 2001;27:361-3.
[Google Scholar]
|
| 152. |
Kaur S, Kanwar AJ. Folliculitis decalvans: Successful treatment with a combination of rifampicin and topical mupirocin. J Dermatol 2002;29:180-1.
[Google Scholar]
|
| 153. |
Abeck D, Korting HC, Braun-Falco O. Folliculitis decalvans. Longlasting response to combined therapy with fusidic acid and zinc. Acta Derm Venereol (Stockh) 1992;72:143-5.
[Google Scholar]
|
| 154. |
Karakuzu A, Erdem T, Aktas A. A case of folliculitis decalvans involving the beard, face and nape. J Dermatol 2001;28:329-31.
[Google Scholar]
|
| 155. |
Paquet P, Pierard GE. Dapsone treatment of folliculitis decalvans. Ann Dermatol Venereol 2004;131:195-7.
[Google Scholar]
|
| 156. |
Farhi D, Buffard V, Ortonne N. Tufted folliculitis of the scalp and treatment with cyclosporine. Arch Dermatol 2006;142:251-2.
[Google Scholar]
|
| 157. |
Parlette EC, Kroeger N, Ross EV. Nd: YAG laser treatment of recalcitrant folliculitis decalvans. Dermatol Surg 2004;30:1152-4.
[Google Scholar]
|
| 158. |
Smith EP, Hardaway CA, Graham BS. Folliculitis decalvans treated with radiation therapy. Cutis 2006;78:162-4.
[Google Scholar]
|
| 159. |
Goo B, Chung HJ, Chung WG. Intramuscular immunoglobulin for recalcitrant suppurative diseases of the skin: A retrospective review of 63 cases. Br J Dermatol 2007;157:563-8.
[Google Scholar]
|
| 160. |
Dhaoui MA, Mebazaa A, Doss N. Dissecting cellulitis of the scalp: Treatment by isotretinoin. Ann Dermatol Venereol 2001;128:688.
[Google Scholar]
|
| 161. |
Scheinfeld NS. A case of dissecting cellulitis and a review of the literature. Dermatol Online J 2003;9:8.
[Google Scholar]
|
| 162. |
Shaffer N, Billick RC, Srolovitz H. Perifolliculitis capitis abscedens et suffodiens. Resolution with combination therapy. Arch Dermatol 1992;128:1329-31.
[Google Scholar]
|
| 163. |
Salim A, David J, Holder J. Dissecting cellulitis of the scalp with associated spondylarthropathy: case report and review. J Eur Acad Dermatol Venereol 2003;17:689-91.
[Google Scholar]
|
| 164. |
Karpouzis A, Giatromanolaki A, Sivridis E. Perifolliculitis capitis abscedens et suffodiens successfully controlled with topical isotretinoin. Eur J Dermatol 2003;13:192-5.
[Google Scholar]
|
| 165. |
Bellew SG, Nemerofsky R, Schwartz RA. Successful treatment of recalcitrant dissecting cellulitis of the scalp with complete scalp excision and split-thickness skin graft. Dermatol Surg 2003;29:1068-70.
[Google Scholar]
|
| 166. |
Bachynsky T, Antonyshyn OM, Ross JB. Dissecting folliculitis of the scalp: A case report of combined treatment using tissue expansion, radical excision, and isotretinoin. J Dermatol Surg Oncol 1992;18:877-80.
[Google Scholar]
|
| 167. |
Boyd AS, Binhlam JQ. Use of an 800-nm pulsed-diode laser in the treatment of recalcitrant dissecting cellulitis of the scalp. Arch Dermatol 2002;138:1291-3.
[Google Scholar]
|
| 168. |
Chinnaiyan P, Tena LB, Brenner MJ, Welsh JS. Modern external beam radiation therapy for refractory dissecting cellulitis of the scalp. Br J Dermatol 2005;152:777-9.
[Google Scholar]
|
| 169. |
Krasner BD, Hamzavi FH, Murakawa GJ, Hamzavi IH. Dissecting cellulitis treated with the long-pulsed Nd: YAG laser. Dermatol Surg 2006;32:1039-44.
[Google Scholar]
|
| 170. |
Mahe A. Treatment of acne keloidalis nuchae: recommendations. Ann Dermatol Venereol 1999;126:541-2.
[Google Scholar]
|
| 171. |
Gloster HM Jr. The surgical management of extensive cases of acne keloidalis nuchae. Arch Dermatol 2000;136:1376-9.
[Google Scholar]
|
| 172. |
Shah GK. Efficacy of diode laser for treating acne keloidalis nuchae. Indian J Dermatol Venereol Leprol 2005;71:31-4.
[Google Scholar]
|
| 173. |
Petiau P, Cribier B, Chartier C. Acne necrotica varioliformis: Resolution with isotretinoin. Eur J Dermatol 1994;4:608-10.
[Google Scholar]
|
| 174. |
Séez M, Rodríguez-Martín M, Sidro M, Carnerero A, García-Bustínduy M, Noda A. Successful treatment of erosive pustular dermatosis of the scalp with topical tacrolimus. Clin Exp Dermatol 2005;30:599-600.
[Google Scholar]
|
| 175. |
Boffa MJ. Erosive pustular dermatosis of the scalp successfully treated with calcipotriol cream. Br J Dermatol 2003;148:593-5.
[Google Scholar]
|
| 176. |
Jiang C, Ting AT, Seed B. PPAR-gamma agonists inhibit production of monocyte inflammatory cytokines. Nature 1998;391:82-6.
[Google Scholar]
|
| 177. |
Ricote M, Li AC, Willson TM, Kelly CJ, Glass CK. The peroxisome proliferator activated receptor-gamma is a negative regulator of macrophage activation. Nature 1998;391:79-82.
[Google Scholar]
|
| 178. |
Kwon OS, Kim MH, Park SH, Chung JH, Eun HC, Oh JK. Staged hair transplantation in cicatricial alopecia after carbon dioxide laser-assisted scar tissue remodeling. Arch Dermatol 2007;143:457-60.
[Google Scholar]
|
| 179. |
Podda M, Spieth K, Kaufmann R. Er: YAG laser-assisted hair transplantation in cicatricial alopecia. Dermatol Surg 2000;26:1010-4.
[Google Scholar]
|
Fulltext Views
16,939
PDF downloads
5,079





