Translate this page into:
Prevalence and antimicrobial susceptibility of Ureaplasma urealyticum and Mycoplasma hominis in Chinese women with genital infectious diseases
2 Department of Respiratory Medicine, Shanghai Pulmonary Hospital, Tongji University School of Medicine, Shanghai, China
3 Department of Oncological Medicine, Changzhou Tumor Hospital Soochow University, Changzhou, China
4 Department of Gynaecology, Changzhou Tumor Hospital Soochow University, Changzhou, China
Correspondence Address:
Yang Ling
Department of Oncological Medicine, Changzhou Tumor Hospital Soochow University, Changzhou 213001
China
| How to cite this article: Zhu C, Liu J, Ling Y, Dong C, Wu T, Yu X, Hou Y, Dong L, Cheng X. Prevalence and antimicrobial susceptibility of Ureaplasma urealyticum and Mycoplasma hominis in Chinese women with genital infectious diseases. Indian J Dermatol Venereol Leprol 2012;78:406-407 |
Abstract
Background: Previous studies have confirmed that the presence of Ureaplasma urealyticum (UU) and Mycoplasma hominis (MH) increases the risks of various diseases including genital infections in women. Hence, the surveillance policies for the prevalence and antimicrobial susceptibility of UU and MH are important for both the prevention and the treatment of the diseases associated with genital mycoplasmas. Aim: The objective of this study is to investigate the characteristics of UU and MH prevalence and its antimicrobial susceptibility in Chinese women with genital infection. Methods: By using commercial mycoplasma strips, we investigated the incidence and antimicrobial resistance of UU and MH in 3306 Chinese women with genital infection between January 2005 and December 2009 in Changzhou China. Results: (1) The overall positive incidence of genital mycoplasmas was 62.16%. The most common pattern was UU monoinfection (46.52%), the UU-MH coinfection pattern ranked second (13.91%) and MH monoinfection was lowest (1.71%). According to annual analysis, MH infection revealed an increasing trend between 2005 and 2009. However, a significantly higher infection rate by genital mycoplasmas was found in young women (age range: 16-35 years). (2) Overall, MH susceptibility rates remained high only to doxycycline (DOX), minocycline (MIN) and josamycin (JOS), while UU had high susceptibility rates only to DOX, MIN and clarithromycin (CLA). The resistance rates of UU-MH-mixed isolates to most of drugs were significantly higher than those of UU- or MH-single isolates. Conclusions: High infection rates and severe drug resistances of genital mycoplasmas were found in Chinese women with genital infections. The laboratory screening and antimicrobial susceptibility testing for genital mycoplasmas is vital to treat the infection.Introduction
Ureaplasma urealyticum (UU) and Mycoplasma hominis (MH) belong to the Ureaplasma group of microorganisms. They are the smallest free-living organisms and are unique among prokaryotes in that they lack a cell wall. Although it is an opportunistic pathogen, UU can produce some toxic substances such as urease, IgA protease and phospholipase enzymes and colonize through adhesion in the respiratory tract or urinary tract epithelial cell surface receptors. [1] This increases the risk of some infectious diseases, including nongonococcal urethritis, [2],[3] prostatitis, [4] testicular inflammation, [5],[6] urinary stones, [7] infertility, [8],[9],[10] preterm delivery, [11] low birth weight in newborn, [12] chronic lung disease of newborns or infants [13],[14],[15] and neonatal respiratory distress syndrome. [16] Moreover, MH is similar to UU in regards to pathogenicity. Presently, UU is divided into two groups (Ureaplasma Parvum, biovar 1, parvo) and (Ureaplasma urealyticum, biovar 2, T960). Biovar 1 includes serotypes 1, 3, 6 and 14, and the remaining 10 serotypes belong to biovar 2. Previous studies have revealed that UU biovar and serotype may be associated with pathogenicity and resistance. [17],[18],[19],[20]
At present, genital tract infections are common diseases in Chinese women. There are many studies that demonstrated that in women some genital tract infections such as vaginitis, cervicitis and pelvic inflammatory disease are associated with UU and MH infection. [21],[22],[23],[24] Therefore, the surveillance programs for monitoring the prevalence and antimicrobial susceptibility of UU and MH are important for both the prevention and the treatment of UU and MH infections. In this present study, we attempted to investigate the characteristics of the prevalence and the antimicrobial susceptibility of UU and MH in Chinese women with genital infection.
Methods
Study participants
The study group consisted of 3306 consecutive female patients with genital tract infection admitted to the Gynaecological Department of Changzhou Fourth Hospital Soochow University and Changzhou Tumor Hospital between January 2005 and December 2009. The median age of the patients was 27 years (range: 17-68 years). The infection types mainly included vaginitis, cervicitis, vulvitis, cystitis, salpingitis as well as pelvic inflammatory disease and so on. The clinical diagnoses were independently conducted by the gynecologists according to the guidelines for diagnosis and treatment of genitourinary tract infectious diseases. The participants who agreed to this project were included in this present study. Patients with a mixed infection by Neisseria gonorrhoeae, Chlamydia trachomatis, Trichomoniasis and Candida and those who received antimicrobial agents within 7 days were excluded from the study. In this study, the gynecologists were blind to the results of the assays and the laboratory staff were blind to the diagnosis of the patients as well. All these patients were treated in accordance with the Helsinki Declaration regarding the participation of human subjects in medical research. The study was approved by the Soochow University Bioethics Committee.
Mycoplasmas detection and antimicrobial susceptibility testing
The samples for testing genital mycoplasmas (UU and MH) were collected by picking up endocervical cells or vaginal secretion using sterile swabs. The culture of genital mycoplasmas was performed by using a commercial mycoplasma strip (Zhuhai Lizhu Bio-Company China, Guangzhou, China). The principle of the strip is as follows: Specific enzymolysis of UU for urea and MH for 2-amino-5-guanidinovaleric acid in medium produces ammonia, which turns the medium red due to the presence of phenol red, which was previously added to the medium. Structurally, the strip has 24 test wells (negative control well, positive control well, UU identification well, UU quantification well, MH identification well, MH quantification well, nine wells containing low-concentration drugs for nine kinds of antimicrobials, nine wells containing high-concentration drugs for nine kinds of antimicrobials). Therefore, this test strip allows identification of genital mycoplasmas within 48 h and estimation of the amount of mycoplasma colonization or infection (>10 4 color change unit is regarded as evidence of infection); at the same time, the strips can also detect the antimicrobial susceptibilities.
The results of resistance or susceptibility to antimicrobial agents in the strips were judged based on the guidelines of the Clinical and Laboratory Standards Institute (CLSI). The breakpoints for the antimicrobials tested are as follows (mg/L): doxycycline (DOX) S≤4, R≥8; minocycline (MIN) S≤1, R≥4; josamycin (JOS) S≤2, R≥8; roxithromycin (ROX) S≤1, R≥4; clarithromycin (CLA) S≤1, R≥4; azithromycin (AZI) S≤0.12, R≥4; ofloxacin (OFL) S≤1, R≥4; levofloxacin (LEV) S≤1, R≥4; sparfloxacin (SPA) S≤1, R≥4.
Statistical analysis
SPSS 11.5 software (Statistical Package for the Social Sciences) was used to analyze the data. Pearson Chi-square test or Fisher exact test was used to assess categorical variables and the linear regression test and logistic regression test were used to verify a trend analysis. P<0.05 was considered statistically significant.
Results
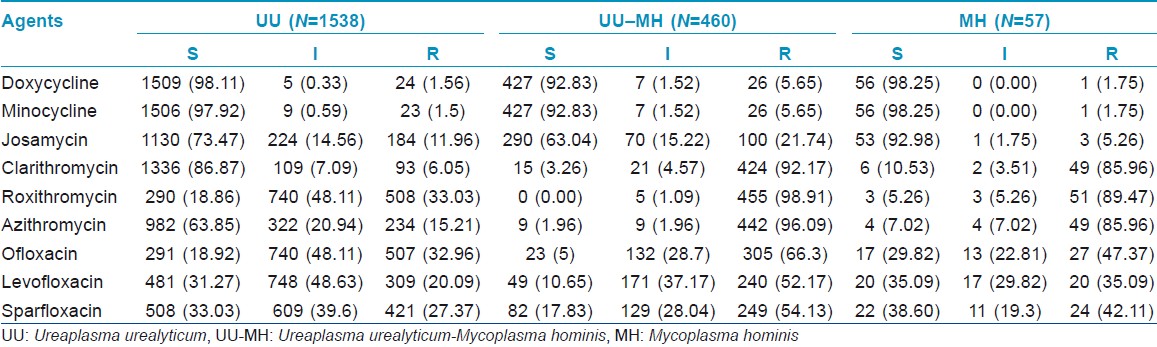
In 3306 females with genital infectious diseases, the positive rates of UU monoinfection, UU-MH coinfection and MH monoinfection were 46.2%, 13.91% and 1.72%, respectively. The overall UU- and MH-positive rate was 62.16%. Annual analysis showed that UU annual incidences were distinctly higher than MH incidences, and regression analyses indicated that UU-MH coinfection increased between 2005 and 2009 (linear correlation coefficient: 0.957, P<0.05; logistic correlation coefficient: 0.822, P<0.05) [Table - 1] and [Figure - 1]. There was a significantly higher infection rate of genital mycoplasmas in young women (age range: 16-35 years) than that in the elder women group (P<0.05) [Table - 2].


 |
| Figure 1: Trend of the 5 - year incidences of Ureaplasma urealyticum and Mycoplasma hominis in Chinese women with genital infectious diseases |
The resistance rates of UU-MH-mixed isolates to nine kinds of drugs were significantly higher than that of UU single isolates (P<0.05). Compared with MH single isolates, apart from DOX and MIN, the resistance rates of UU-MH-mixed isolates to the other seven kinds of drugs were higher (P<0.05). CLA-, ROX- and AZI-resistance rates in MH were higher than that in UU (P<0.05). UU to DOX, MIN and CLA and MH to DOX, MIN and JOS kept high susceptibility rates [Table - 3] and [Table - 4]. Both UU and MH had high resistance rates to quinolones (OFL, LEV and SPA).

Discussion
Characteristics of genital mycoplasma infection
According to previous large sample studies published in other provinces of China, the overall positive rates for genital mycoplasmas in Chinese women with genital infection ranged from 45.8% to 66.5%. In this present study, we confirmed that Chinese women with genital infection had a high genital mycoplasma rate (62.16%) and that the most common infection pattern was UU monoinfection (46.52%). The second cause was UU-MH coinfection pattern (13.91%) and MH monoinfection was lowest (1.71%), which was consistent with the results reported in other provinces of China.
However, an important transmission route of UU and MH is via sexual contact; therefore, active sexual behavior in populations might contribute to the increasing risks of UU and MH infection. Previous studies showed that UU and MH infections were most frequently found in the group of patients attending an STD clinic, and correlated with age and sexual activity. [25] In this study, we also confirmed that a significantly higher infection rate is found in the young women (age range: 16-35 years) than in the elder women group. The overall incidences of sexually transmitted infections (STIs) in Chinese populations have been increasing continuously during the past three decades. According to the report by Chen et al., [26] there was an estimated 2.8-4.5 million female sex workers (FSWs) and 21.9-37.4 million clients for FSWs, 2-10 million sex with men (MSM) and 130 million migrant sex workers in China. Human immunodeficiency virus/acquired immunodeficiency syndrome and other STIs has been spreading from high-risk populations to the general population. The reported incidence of primary and secondary syphilis (reflecting the intensity of recent transmission) was 11.7 cases per 100,000 residents in 2009, which increased by 2.1-times since 2005. However, lack of timely check-ups and proper treatment was also another important factor causing high rates of genital mycoplasma infection. In fact, there was only a small proportion of women with genital infection who could afford to attend the special clinics for screening mycoplasmas and antimicrobial susceptibility testing in China. Some prescriptions for treating genital infection were based on physicians′ empirical treatments rather than laboratory evidences, and this not only led to failure of treatment against mycoplasmas but also caused antimicrobial agent abuse. All these factors put together caused a high mycoplasma infection rate in Chinese women.
In addition, our study showed that the UU-MH coinfection pattern predominated in MH infection cases while MH monoinfection was rare. This suggests that MH is less likely to colonize alone and that UU colonization seems to contribute to the secondary MH infection. We postulated that the alkalization of genital tract caused by UU colonization might be a promoting factor for the growth of MH.
From 2005 to 2009, the overall MH infection rate revealed an upward trend. What caused this elevation of MH infection rate? We postulated that one of the important reasons was that macrolides, including AZI, ROX and CLA, had been widely used to treat the genital infectious diseases for a long time in this region. However, according to our studies, macrolides did not have any activity against MH; and, naturally, MH infection incidences appeared to increase under the selective antimicrobial pressures. Considering that the pattern of the antimicrobial use in this region is less likely to be reversed in the future, we believe that the MH infection rate will continue to increase. We also believe that conducting screening and antimicrobial susceptibility testing for mycoplasmas in women with genital infectious diseases is vital so as to avoid antimicrobial abuse and to curb the elevation due to MH infection.
Characteristics of antimicrobial resistance of Ureaplasma urealyticum and Mycoplasma hominis
Because of the lack of a cell wall, UU and MH are naturally resistant to beta-lactam antimicrobial agents. Currently, three kinds of antimicrobial agents including quinolones, tetracyclines and macrolides are commonly used for treating mycoplasma infections. However, mycoplasmas can be resistant to the above antimicrobials due to the emergence of different resistance mechanisms. Mycoplasma resistance to macrolides occurs when the latter is used as an inducer of methylase and causes the methylation of the 50 S ribosomal large subunit. [27] The resistance to quinolones is mainly due to the mutation of the target enzyme-DNA helicase, [28],[29] and the resistance mechanism of tetracyclines involves tetM genes by a Tn1545-like transposon. [30],[31],[32]
Based on our results, only tetracyclines can retain its higher antibacterial activities against genital mycoplasmas, while quinolones and macrolides revealed poor activities in this region. In detail, UU had higher susceptibility to DOX, MIN and CLA; moderate susceptibility to JOS and AZI; while it had very low susceptibility to ROX, OFL, LEV and SPA. Susceptibility of MH to DOX, MIN and JOS remained high, while it kept low susceptibilities to CLA, ROX and AZI in addition to quinolones (OFL, LEV and SPA). At the same time, our investigation also showed that the resistance rates of UU-MH-mixed isolates to most of drugs were higher when it was compared with that of UU or MH single isolates, indicating that the UU-MH coinfection is difficult to treat.
Like other bacteria, the antimicrobial susceptibility of mycoplasmas had a distinct geographic feature. The causes of geographic differences in resistance were complex. We believe that one of the main reasons is the differences in the frequencies of antimicrobials used in different regions. In fact, quinolones and macrolides were more commonly used for treating MH and UU infection in Changzhou China. According to our survey, the sensitivities of quinolones and macrolides for UU and MH have declined compared with an earlier stage in China.
Considering the high-antimicrobial resistance of MH and UU in Chinese females with genital infectious diseases, conducting antimicrobial susceptibility testing is an important and necessary step for using appropriate antimicrobials. In addition, we have detected some extensive drug-resistant UU and MH clinical strains (resistance to all nine kinds of drugs), which gave rise to the difficulties in the treatment of mycoplasmas infection in terms of currently available antimicrobials. Fortunately, some Chinese herbs such as Galla Chinensis, Fructus Forsythiae and Cortex Phellodendrim and so on were reported to have antibiotic activity against UU in vitro. These are promising alternatives that can be used to treat UU infectious diseases. [33],[34]
In brief, high infection rates and severe drug resistances of UU and MH were found in Chinese women with genital infectious diseases. The laboratory screening and antimicrobial susceptibility testing for genital mycoplasmas is vital to treat these infections.
| 1. |
Smith DG, Russell WC, Thirkell D. Adherence of Ureaplasma urealyticum to human epithelial cells. Microbiology 1994;140:2893-8.
[Google Scholar]
|
| 2. |
Couldwell DL, Gidding HF, Freedman EV, McKechnie ML, Biggs K, Sintchenko V, et al. Ureaplasma urealyticum is significantly associated with non-gonococcal urethritis in heterosexual Sydney men. Int J STD AIDS 2010;21:337-41.
[Google Scholar]
|
| 3. |
Iser P, Read TH, Tabrizi S, Bradshaw C, Lee D, Horvarth L, et al. Symptoms of non-gonococcal urethritis in heterosexual men: A case control study. Sex Transm Infect 2005;81:163-5.
[Google Scholar]
|
| 4. |
Radoniæ A, Kovaceviæ V, Markotiæ A, Skerk V, Turciæ P, Skerk V. The clinical significance of Ureaplasma urealyticum in chronic prostatitis. J Chemother 2009;21:465-6.
[Google Scholar]
|
| 5. |
Malewa E, Dimitrowa C. Experimental orchitis in white rats, caused by Ureaplasma urealyticum. Zentralbl Allg Pathol 1983;128:391-7.
[Google Scholar]
|
| 6. |
Marsaudon E, Moulin P, Anstett-Barrault MF. Discovery of testicular microlithiasis in the course of Ureaplasma urealyticum urethritis. Rev Med Interne 2000;21:201-3.
[Google Scholar]
|
| 7. |
Reyes L, Reinhard M, O'donell LJ, Stevens J, Brown MB. Rat strains differ in susceptibility to Ureaplasma parvum-induced urinary tract infection and struvite stone formation. Infect Immun 2006;74:6656-64.
[Google Scholar]
|
| 8. |
Gdoura R, Kchaou W, Ammar-Keskes L, Chakroun N, Sellemi A, Znazen A, et al. Assessment of Chlamydia trachomatis, Ureaplasma urealyticum, Ureaplasma parvum, Mycoplasma hominis, and Mycoplasma genitalium in semen and first void urine specimens of asymptomatic male partners of infertile couples. J Androl 2008;29:198-206.
[Google Scholar]
|
| 9. |
Gdoura R, Kchaou W, Chaari C, Znazen A, Keskes L, Rebai T, et al. Ureaplasma urealyticum, Ureaplasma parvum, Mycoplasma hominis and Mycoplasma genitalium infections and semen quality of infertile men. BMC Infect Dis 2007;7:129.
[Google Scholar]
|
| 10. |
Liu DF, Jiang H, Hong K, Zhao LM, Tang LF, Liu JM, et al. A meta-analysis of Ureaplasma urealyticum infection and Chinese male infertility. Zhonghua Nan Ke Xue 2008;14:618-23.
[Google Scholar]
|
| 11. |
Kacerovsky M, Boudys L. Preterm premature rupture of membranes and Ureaplasma urealyticum. Ceska Gynekol 2008;73:154-9.
[Google Scholar]
|
| 12. |
Kirchner L, Helmer H, Heinze G, Wald M, Brunbauer M, Weninger M, et al. Amnionitis with Ureaplasma urealyticum or other microbes leads to increased morbidity and prolonged hospitalization in very low birth weight infants. Eur J Obstet Gynecol Reprod Biol 2007;134:44-50.
[Google Scholar]
|
| 13. |
Honma Y, Yada Y, Takahashi N, Momoi MY, Nakamura Y. Certain type of chronic lung disease of newborns is associated with Ureaplasma urealyticum infection in utero. Pediatr Int 2007;49:479-84.
[Google Scholar]
|
| 14. |
Yada Y, Honma Y, Koike Y, Takahashi N, Momoi MY. Association of development of chronic lung disease of newborns with neonatal colonization of Ureaplasma and cord blood interleukin-8 level. Pediatr Int 2010;52:718-22.
[Google Scholar]
|
| 15. |
Pandey A, Dhawan B, Gupta V, Chaudhry R, Deorari AK. Clinical significance of airways colonization with Ureaplasma urealyticum in premature (<34 wk) neonates. Indian J Med Res 2007;125:679-84.
[Google Scholar]
|
| 16. |
Cultrera R, Seraceni S, Germani R, Contini C. Molecular evidence of Ureaplasma urealyticum and Ureaplasma parvum colonization in preterm infants during respiratory distress syndrome. BMC Infect Dis 2006;6:166.
[Google Scholar]
|
| 17. |
Ekiel AM, Friedek DA, Romanik MK, JóŸwiak J, Martirosian G. Occurrence of Ureaplasma parvum and Ureaplasma urealyticum in women with cervical dysplasia in Katowice, Poland. J Korean Med Sci 2009;24:1177-81.
[Google Scholar]
|
| 18. |
De Francesco MA, Negrini R, Pinsi G, Peroni L, Manca N. Detection of Ureaplasma biovars and polymerase chain reaction-based subtyping of Ureaplasma parvum in women with or without symptoms of genital infections. Eur J Clin Microbiol Infect Dis 2009;28:641-6.
[Google Scholar]
|
| 19. |
Yoshida T, Deguchi T, Meda S, Kubota Y, Tamaki M, Yokoi S, et al. Quantitative detection of Ureaplasma parvum (biovar 1) and Ureaplasma urealyticum (biovar 2) in urine specimens from men with and without urethritis by real-time polymerase chain reaction. Sex Transm Dis 2007;34:416-9.
[Google Scholar]
|
| 20. |
Chang-Tai Z, Zhong-Yi H, Chun-Lei D, Chang-Song Z, Mei-Zhen W, Yang L. Investigation of Ureaplasma urealyticum biovars and their relationship with antimicrobial resistance. Indian J Med Microbiol 2011;29:288-92.
[Google Scholar]
|
| 21. |
Rar VA, Maksimova TG, Trukhina AV, Rempel' EG, Klimashevskaia NI, Riabova EN, et al. Level of colonization by Ureaplasma urealyticum of definite biovars in a group of women with different clinical symptoms. Zh Mikrobiol Epidemiol Immunobiol 2004:12-7.
et al. Level of colonization by Ureaplasma urealyticum of definite biovars in a group of women with different clinical symptoms. Zh Mikrobiol Epidemiol Immunobiol 2004:12-7.'>[Google Scholar]
|
| 22. |
Potts JM, Ward AM, Rackley RR. Association of chronic urinary symptoms in women and Ureaplasma urealyticum. Urology 2000;55:486-9.
[Google Scholar]
|
| 23. |
Elias M, Grze?ko J, Siejkowski R, Nowicka J, Maczyñska B, Goluda M, et al. The presence of Mycoplasma hominis and Ureaplasma urealyticum in the cervical canal of uterus. Ginekol Pol 2005;76:28-32.
[Google Scholar]
|
| 24. |
Keane FE, Thomas BJ, Gilroy CB, Renton A, Taylor-Robinson D. The association of Mycoplasma hominis, Ureaplasma urealyticum and Mycoplasma genitalium with bacterial vaginosis: Observations on heterosexual women and their male partners. Int J STD AIDS 2000;11:356-60.
[Google Scholar]
|
| 25. |
Zdrodowska-Stefanow B, K³osowska WM, Ostaszewska-Puchalska I, Bu³hak-Kozio³ V, Kotowicz B. Ureaplasma urealyticum and Mycoplasma hominis infection in women with urogenital diseases. Adv Med Sci 2006;51:250-3.
[Google Scholar]
|
| 26. |
Chen XS, Peeling RW, Yin YP, Mabey DC. The epidemic of sexually transmitted infections in China: Implications for control and future perspectives. BMC Med 2011;9:111.
[Google Scholar]
|
| 27. |
Felmingham D, Robbins MJ, Sanghrajka M, Leakey A, Ridgway GL. The in vitro activity of some 14-, 15- and 16- membered macrolides against Staphylococcus spp., Legionella spp., Mycoplasma spp. and Ureaplasma urealyticum. Drugs Exp Clin Res 1991;17:91-9.
[Google Scholar]
|
| 28. |
Duffy L, Glass J, Hall G, Avery R, Rackley R, Peterson S, et al. Fluoroquinolone resistance in Ureaplasma parvum in the United States. J Clin Microbiol 2006;44:1590-1.
[Google Scholar]
|
| 29. |
Zhang W, Wu Y, Yin W, Yu M. Study of isolation of fluoroquinolone-resistant Ureaplasma urealyticum and identification of mutant sites. Chin Med J (Engl) 2002;115:1573-5.
[Google Scholar]
|
| 30. |
De Barbeyrac B, Dupon M, Rodriguez P, Renaudin H, Bébéar C. A Tn1545-like transposon carries the tet(M) gene in tetracycline resistant strains of Bacteroides ureolyticus as well as Ureaplasma urealyticum but not Neisseria gonorrhoeae. J Antimicrob Chemother 1996;37:223-32.
[Google Scholar]
|
| 31. |
Beeton ML, Chalker VJ, Maxwell NC, Kotecha S, Spiller OB. Concurrent titration and determination of antibiotic resistance in ureaplasma species with identification of novel point mutations in genes associated with resistance. Antimicrob Agents Chemother 2009;53:2020-7.
[Google Scholar]
|
| 32. |
Soroka AE, Akopian TA, Taraskina AE, Baĭtsur MV, Savicheva AM, Govorun VM. Allelic polymorphism of the tem(M) determinant in Mycoplasma hominis and Ureaplasma urealyticum clinical isolates resistant to tetracyclines. Genetika 2002;38:1463-9.
[Google Scholar]
|
| 33. |
Wei H, Chen Z, Xu P, Ma YG, Xu LJ. Effect of Jieze No.1 on cervicitis caused by Ureaplasma urealyticum and on ureaplasma urealyticum in vitro. Chin J Integr Med 2008;14:88-93.
[Google Scholar]
|
| 34. |
Zhu C, Dong C, Kong Y, Liu L, Wu Q, Yao Y. Microdilution inhibition test of Chinese herbs to assess their effect against clinical strains of Ureaplasma urealyticum in vitro. J Nanjing Med Univ 2009;23:143-5.
[Google Scholar]
|
Fulltext Views
4,957
PDF downloads
3,172





