Translate this page into:
Therapeutic efficacy and safety of propylthiouracil in psoriasis: An open-label study
2 Department of Medical Research, SRM Medical College Hospital and Research Centre, Kattankulathur, Kancheepuram District, Tamil Nadu, India
3 Assistant Professor of Dermatology, SRM Medical College Hospital and Research Centre, Kattankulathur, Kancheepuram District, Tamil Nadu, India
Correspondence Address:
Pushpa Gnanaraj
A 1/2, Aishwaryam Phase I, 4th Main Road, Nolumbur, Mogappair West, Chennai - 600 095, Tamilnadu
India
| How to cite this article: Gnanaraj P, Malligarjunan H, Dayalan H, Karthikeyan S, Narasimhan M. Therapeutic efficacy and safety of propylthiouracil in psoriasis: An open-label study. Indian J Dermatol Venereol Leprol 2011;77:673-676 |
Abstract
Background: Psoriasis is a common hyperproliferative disorder of the skin associated with significant morbidity. Most of the drugs used in psoriasis provide only a temporary relief, whereas they are riddled with potential toxicities and cost concerns. Hence, there is a constant need to explore newer, effective, orally administered, and cost-effective drugs with minimal adverse effects. In this scenario, propylthiouracil (PTU), an antithyroid thioureylene has been shown to be effective in psoriasis which satisfies the above criteria. Aim: The objective of our study is to assess the clinical efficacy of PTU in psoriasis. Methods: A total of 25 patients with plaque psoriasis were treated with oral PTU for 12 weeks. Clinical response was assessed using the "Psoriasis Area and Severity Index" (PASI) score. Routine blood analyses and thyroid function tests were carried out periodically during the study. Results: Oral PTU produced significant clearing of lesions at 6 weeks and 12 weeks of the study period in all patients, as demonstrated by the reduction in PASI scores (33.9% in 6 weeks and 74.1% reduction in 12 weeks). Four patients experienced near complete clearing of the lesions. One patient developed mild elevation of liver enzymes which reversed on withdrawal of PTU. None of the patients had hypothyroidism or cytopenias. Conclusion: PTU significantly clears the lesions in psoriasis with minimal adverse effects. Hence, it can be considered as a therapeutic option in psoriasis, especially when the standard drugs cannot be used due to their toxicities or forbidding cost.Introduction
Psoriasis is a common skin disorder affecting 1 to 3% of the individuals worldwide. It is associated with significant morbidity and degradation of quality-of-life which is comparable with other more serious illnesses. [1]
Many agents are used in the management of this debilitating disease, with the newer "biologicals" being the most recent addition to the therapeutic options of this disorder. But most of the systemic treatment options available for psoriasis are potentially toxic, needing constant monitoring, and/or are very expensive and out of the reach of the common man. Hence, there is a constant need to explore newer therapeutic options for psoriasis which will offer long-term remission, with less toxicity and more affordability to the masses. In this scenario, propylthiouracil (PTU) has emerged as an effective drug in the treatment of psoriasis, with patients showing significant clearance of lesions without major adverse effects. [2]
Methods
The objective of our study was to assess the clinical efficacy of PTU in psoriasis. All patients clinically and histologically diagnosed with chronic stable plaque psoriasis were included in the study. An exhaustive history was taken and all patients underwent detailed physical examination. Their clinical severity was assessed as per "Psoriasis Area and Severity Index" (PASI) score. A total of 25 patients with more than 20% body surface area involvement were included in the study. The exclusion criteria were unstable psoriasis, children, pregnancy, lactation, history of any thyroid disorder and severe systemic illnesses. Also, patients who had received any form of topical or systemic therapy in the previous one month were excluded from the study. Approval from the institutional ethics committee was obtained and all patients signed an informed consent document.
It was an open clinical prospective study over a period of one year, between September 2009 and August 2010. The recruited patients underwent pretreatment baseline investigations like complete blood counts, blood sugars, liver, renal and thyroid function tests. A skin biopsy was done at the beginning of the study. The patients were then administered oral PTU 100 mg, three times a day for 12 weeks. They did not receive any other drug apart from topical liquid paraffin as emollient. The patients were assessed at the end of 2, 6, and 12 weeks. At each visit, clinical assessment was done and the PASI score was recorded by the same observer. Adverse reactions, if any, were noted. The blood parameters were repeated at the end of 6 and 12 weeks.
All statistical evaluation was done using SPSS version 13.0. The results are expressed as mean ± SD. Student′s paired ′t′ test was used for statistical analysis of the data. Level of significance was P<0.05.
Results
Of the 25 patients included in the study, 15 were men and 10 were women (mean ± SD, 44.3 ± 11.8 years; range, 19 to 73 years). PTU was well tolerated and accepted by nearly all the patients. Two patients, both men, dropped out of the study due to the following reasons: one patient shifted to an indigenous therapy in the second week of treatment for unknown reasons and the other showed mild elevation of liver enzymes (which reverted to normal on cessation of PTU). The drug was well tolerated in the remaining patients, 13 men and 10 women. The mean PASI scores at the beginning of the study were 17.86 ± 9.9. At the end of 2 weeks, the PASI reduction was not significant (14.36 ± 12.6, P = 0.65). From then on, the PASI scores showed a steady decline with the values being 11.81 ± 10.0 (P = 0.04) at the end of 6 weeks. Finally, at the end of the study period of 12 weeks, the PASI scores were significantly lower, being 4.63 ± 4.1 [Figure - 1] and [Figure - 2]. The difference was statistically significant with P<0.001 [Figure - 3]. The thyroid function tests remained normal throughout the study period. All the patients were followed up for a further three months after stopping PTU. Four patients developed relapse during this period (17.4%).
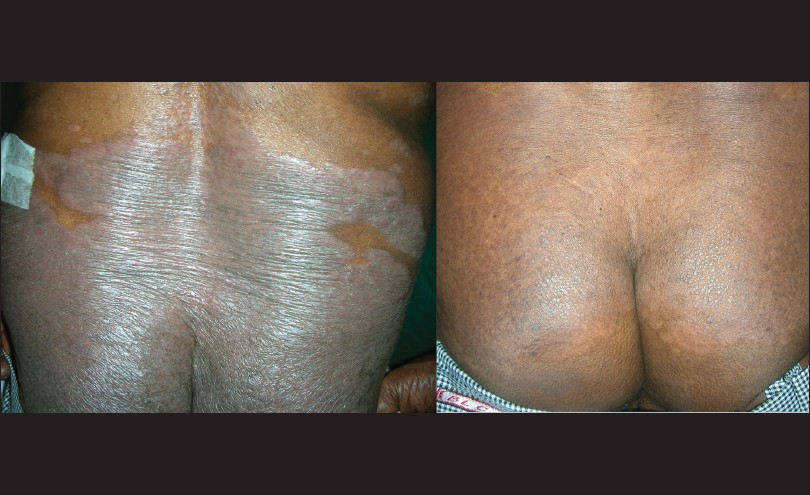 |
| Figure 1: Pictures showing good response to propylthiouracil therapy at the end of 12 weeks |
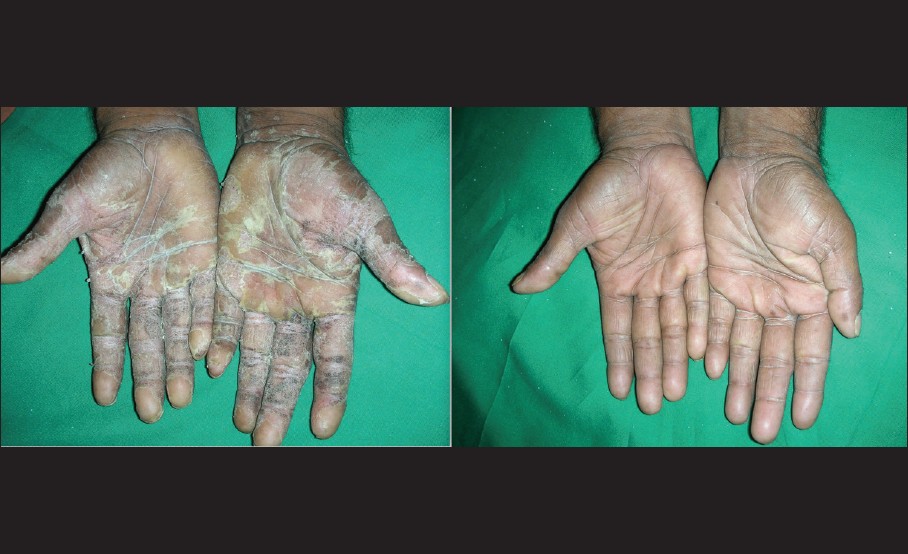 |
| Figure 2: Complete clearance of palmar lesions after 12 weeks of propylthiouracil therapy |
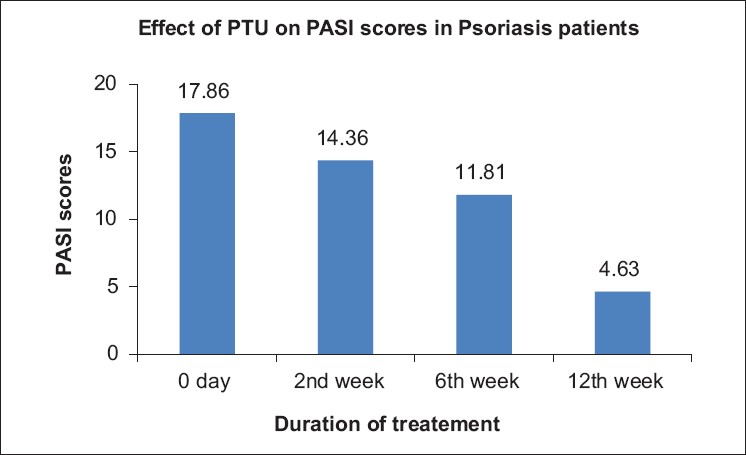 |
| Figure 3: Significant reduction in "Psoriasis Area and Severity Index" scores from 0 to 12 weeks |
Discussion
Psoriasis is a multifactorial disease with various factors involved in its pathogenesis. Hyperproliferation, abnormal differentiation, and activation of the inflammatory mediators are considered the dominant pathogenic mechanisms in psoriasis. In recent years, attention has focused on impaired apoptosis of cells arising from this hyperproliferative event. [3] Overexpression of c-FLIP, an antiapoptotic protein, has been demonstrated in lesional psoriatic skin. [4]
The antithyroid drugs, PTU (6-n-propyl-2-thiouracil), and the related thioureylene, methimazole (1-methyl 2-mercaptoimidazole), have been used for decades as first-line treatment for patients with hyperthyroidism. The role of these drugs in the treatment of psoriasis has been addressed for several years. PTU has been shown to cause significant reduction in PASI scores within 4 to 6 weeks of commencement of therapy. [5]
The exact mechanism of action of PTU in psoriasis is yet to be elucidated. PTU and the related thioureylene, methimazole, have been shown to have an antiproliferative effect, as demonstrated by Elias et al,[6] in 1995, who showed that they decrease the expression of proliferative cell nuclear antigen, a marker of cellular proliferation. [6] Thioureylenes also act as free radical scavengers, thus inhibiting oxidative events in the skin, thereby reducing activation of epidermal/dermal lymphocytes which might play vital roles in the initiation of skin lesions in psoriasis. [7] PTU has been found to have no effect on T or B cell activation, as shown by its inability to alter adenosine deaminase activity, ICAM 1 and IL-2 receptor levels, [8],[9],[10] or neither does it reduce CD 1a expression, a marker of differentiated skin antigen presenting cells. [11] PTU also does not seem to act through the pro- and anti-inflammatory cytokines and the serum tumor necrosis factor α. [12]
Recently, Elias and Barr [13] reported that PTU enhanced the apoptosis in psoriatic plaques. [13] But the exact mechanism by which PTU induces apoptosis has not been fully elucidated. PTU has not been found to have any effect on the antiapoptotic proteins like p16 and Bcl-xl. [2],[14] Hence, the probable mechanism of action of PTU in psoriasis has been postulated to be upregulation of apoptosis. Another proposed mechanism is the binding of the T3 receptor resulting in conformational changes which could lead to switching off of genes necessary for keratin synthesis. [15]
Our study shows that PTU significantly clears the lesions in psoriasis. This experience is similar to other studies done earlier by several authors. Elias et al,[5] in 1993 have shown significant clearance of lesions at the end of 8 weeks of treatment with PTU. [5] A similar study done by Chowdhury and Marks [15] in 2001 has shown moderate clinical improvement in 75% of patients at the end of 6 weeks. [15] Kose et al,[8] in 2001 have demonstrated significant clinical response in all their patients at the end of 2 months. [8]
The potential side effects of PTU include hypothyroidism, hepatotoxicity, agranulocytosis, arthralgia, headache, and urticaria. Vasculitis and Anti neutrophil cytoplasmic antibody (ANCA) positivity have also been reported in patients on PTU. [16] In our study, PTU neither induced clinical hypothyroidism, nor did it cause any serious adverse effects like agranulocytosis or acute hepatotoxicity.
The limitations of our study include its small sample size, it being an open-label study and lack of immunohistochemical and biochemical criteria to back up the clinical changes. Also, lack of long-term follow-up of the patients is another limitation to our study.
Hence, we conclude that PTU can be safely included as a treatment option in psoriasis, especially in patients who are resistant to or unable to use the conventional drugs due to their toxicities. Also, PTU can be considered for use in the rotational or sequential therapy in psoriasis along with other drugs. However, the exact mechanism by which PTU acts in psoriasis needs to be further evaluated.
| 1. |
Rapp SR, Feldman SR, Exum ML, Fleischer AB Jr, Reboussin DM. Psoriasis causes as much disability as other major medical diseases. J Am Acad Dermatol 1999;41:401-7.
[Google Scholar]
|
| 2. |
Elias AN. Anti-thyroid thioureylenes in the treatment of psoriasis. Med Hypotheses 2004;62:431-7.
[Google Scholar]
|
| 3. |
Raj D, Brash DE, Grossman D. Keratinocyte apoptosis in epidermal development and disease. J Invest Dermatol 2006;126:243-57.
[Google Scholar]
|
| 4. |
Yang J, Li Y, Liu YQ, Long JW, Tian F, Dong J, et al. Expression of antiapoptotic protein c-FLIP is upregulated in psoriasis epidermis. Eur J Dermatol 2009;19:29-33.
[Google Scholar]
|
| 5. |
Elias AN, Goodman MM, Liem WH, Barr RJ. Propyl thiouracil in psoriasis: Results of an open trial. J Am Acad Dermatol 1993;29:78-81.
[Google Scholar]
|
| 6. |
Elias AN, Barr AJ, Rohan MK, Dangaran K. Effect of orally administered antithyroid thioureylenes on PCNA and P 53 expression in psoriatic lesions. Int J Dermatol 1995;34:280-3.
[Google Scholar]
|
| 7. |
Utas S, Kose K, Yazici C, Akdaº A, Kelestimur F. Antioxidant potential of propylthiouracil in patients with psoriasis. Clin Biochem 2002;35:241-6.
[Google Scholar]
|
| 8. |
Kose K, Utas S, Yazici C, Akdaº A, Kelestimur F. Effect of propylthiouracil on adenosine deaminase activity and thyroid function in patients with psoriasis. Br J Dermatol 2001;144:1121-6.
[Google Scholar]
|
| 9. |
Elias AN, Goodman MM, Rohan MK. Serum ICAM-1 concentrations in patients with psoriasis treated with antithyroid thioureylenes. Clin Exp Dermatol 1993;18:526-9.
[Google Scholar]
|
| 10. |
Elias AN, Goodman MM, Rohan MK. Effect of propylthiouracil and methimazole on serum levels of interleukin-2 receptors in patients with psoriasis. Int J Dermatol 1993;32:537-40.
[Google Scholar]
|
| 11. |
Elias AN, Nanda VS, Barr RJ. CD 1a expression in psoriatic skin following treatment with propylthiouracil, an antithyroid thioureylene. BMC Dermatol 2003;3:3.
[Google Scholar]
|
| 12. |
Elias AN, Nanda VS, Pandian R. Serum TNF- alpha in psoriasis after treatment with propylthiouracil, an antithyroid thioureylene. BMC Dermatol 2001;4:4.
[Google Scholar]
|
| 13. |
Elias AN, Barr RJ. Enhanced apoptosis in psoriatic plaques after treatment with propylthiouracil. Eur J Dermatol 2007;17:353-4.
[Google Scholar]
|
| 14. |
Elias AN, Barr RJ, Nanda VS. p16 expression in psoriatic lesions following therapy with propylthiouracil, an antithyroid thioureylenes. Int J Dermatol 2004;43:889-92.
[Google Scholar]
|
| 15. |
Chowdhury MM, Marks R. Oral propyl thiouracil for the treatment of resistant plaque psoriasis. J Dermatol Treat 2001;12:81-5.
[Google Scholar]
|
| 16. |
Brunton LL, Lazo JS, Parker KL. Goodman and Gilman's The pharmacological Basis of Therapeutics 11 th ed. China: McGraw-Hill Professional 2006. p. 1528-9.
th ed. China: McGraw-Hill Professional 2006. p. 1528-9.'>[Google Scholar]
|
Fulltext Views
3,503
PDF downloads
1,998





