Translate this page into:
Scoring systems in dermatology
Correspondence Address:
Urmila Bhor
Department of Dermatology, Seth GS Medical College and KEM Hospital, Mumbai
India
| How to cite this article: Bhor U, Pande S. Scoring systems in dermatology. Indian J Dermatol Venereol Leprol 2006;72:315-321 |









Dermatologists have the privilege of examining the largest organ of the body. However, unlike other organs, there are hardly any tests of clinical significance that measure skin function. In dermatological practice, methods of evaluating the severity of skin diseases are often crude, subjective and not reproducible, which creates discrepancy in results and inter-individual variations. Hence, to maintain objectivity in observations, scores are used to evaluate the severity of skin diseases. This is particularly important for monitoring the response to therapy and for evaluating the efficacy of new drugs. Over the years scoring systems have been developed for a number of skin diseases. This has greatly helped the cause of clinical practice and clinical research.
This article deals with scores that are commonly used and uniformly accepted for the ease of assessment of common skin diseases.
ATOPIC DERMATITIS
Scores that are commonly used for objective assessment of atopic dermatitis are
1. SCORing Atopic Dermatitis (SCORAD)
2. The Six Area, Six Sign Atopic Dermatitis (SASSAD) severity score
SCORAD (SCORing Atopic Dermatitis)[1]
Developed by the European Task Force on atopic dermatitis in 1993, it is the most commonly used scoring system for measuring the severity of atopic dermatitis. It is used to standardize the assessment of atopic dermatitis and to help in the interpretation of therapeutic studies.
The SCORAD Index is a composite score based on 3 subscores:
A = The extent score based on body surface area calculated using the ′Rule of 9′.
B = Intensity score based on 6 clinical findings in atopic dermatitis, namely erythema, edema or papulations, oozing or crusting, excoriation, lichenification, dryness, graded on a scale of 0 - 3 (0- absent, 1- mild, 2- moderate, 3- severe).
C = The score for pruritus and sleep loss graded on a visual analog scale of 0 to 10. The severity is based on the average extent for the last 3 days or nights.
Final formula for calculation of SCORAD is as follows:
SCORAD = A/5 + 7(B/2) + C
The disadvantage of this scoring system is the significant interobserver variation which makes subsequent assessment of the patient by the same observer necessary.
SASSAD[2]
The Six Area, Six Sign Atopic Dermatitis severity score has proved to be a simple and effective system for recording and monitoring disease activity in atopic dermatitis. The score is obtained by grading six signs (erythema, exudation, excoriation, dryness, cracking and lichenification), each on a scale of 0 (absent), 1 (mild), 2 (moderate), or 3 (severe), at each of six sites (arms, hands, legs, feet, head and neck, trunk). The maximum score is 108.
A modified version of the SASSAD known as the six-area ′total body severity assessment (TBSA)′ has also been described. The TBSA, which has a maximum score of 108, differs from SASSAD in that it assesses infiltration and vesicles and/or papules, and excludes lichenification.[3]
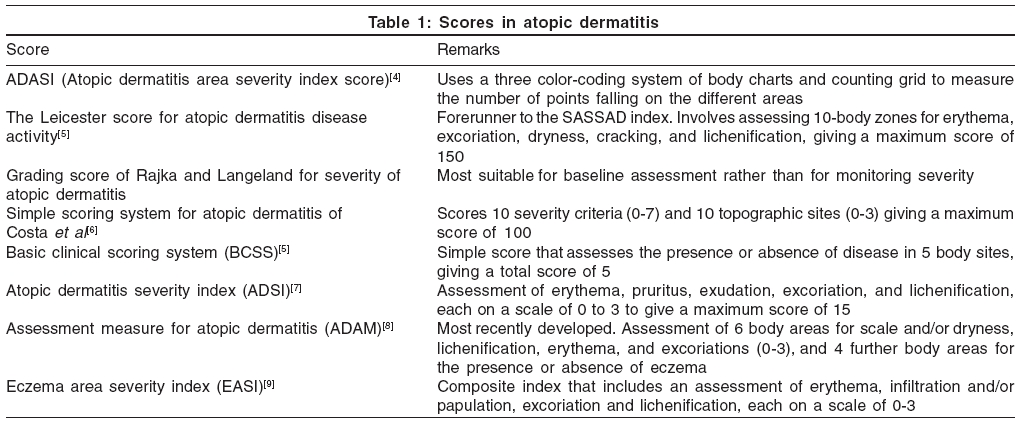
Other scores in AD that are uncommonly used are listed in [Table - 1].
PSORIASIS
Psoriasis area severity index (PASI)[10] This is currently the gold standard score for the assessment of extensive psoriasis, but has the limitation of interobserver variation. Four sites of affection, the head (h), upper limb (u), trunk (t) and lower limbs (l), are separately scored by using three parameters, erythema, induration and desquamation, each of which is graded on a severity scale of 0 to 4, where 0 = nil, 1 = mild, 2 = moderate, 3 = severe and 4 = very severe. The area-wise percentage involvement of the involved sites is calculated as: 1 = less than 10% area; 2 = 10-29%; 3 = 30-49%; 4 = 50-69%; 5 = 70-89%; and 6 = more than 90%.
The final formula for PASI score is:
PASI = 0.1 (Eh + Ih + Dh) Ah + 0.2 (Eu + Iu + Du) Au + 0.3 (Et + It+ Dt) At + 0.4 (El + Il + Dl) Al
The maximum score of PASI is 72. PASI 75 is a 75% reduction of baseline PASI score. It is commonly considered as a denominator for satisfactory results of any treatment modality for psoriasis.
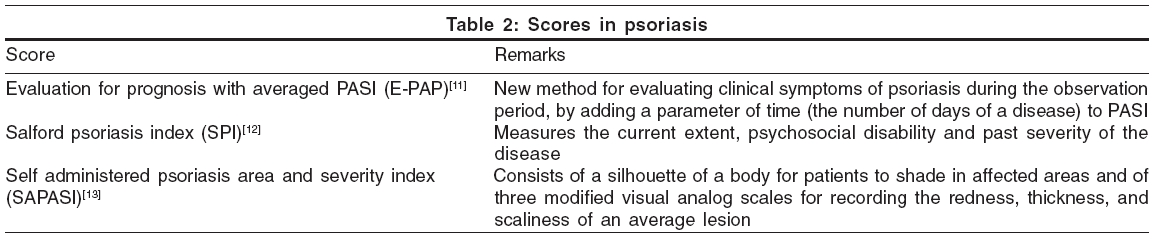
Other scores that are used for psoriasis are stated in [Table - 2].
TOXIC EPIDERMAL NECROSIS (TEN)
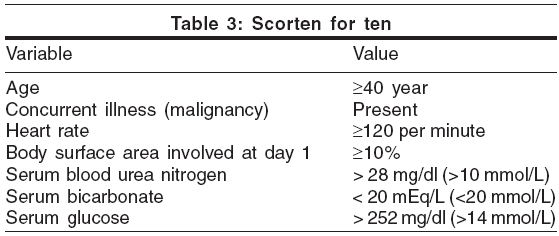
The score commonly used for assessing the patients of TEN is SCORTEN.[14] Scoring is based on the evaluation of seven independent risk factors within the first 24 hours of admission.
One point is assigned to each variable. The value of the total number of points determines the predicted mortality: 0-1 points-3.2% mortality; 2 points-12.1%; 3-35.3%; 4-58.3%; ³5-90%. [Table - 3]
VITILIGO
Two scores designed for the assessment of vitiligo are vitiligo area severity index (VASI)[15] and vitiligo disease activity score (VIDA).[16]
Vitiligo area severity index The percentage of vitiligo involvement is calculated in terms of hand units. One hand unit (which encompasses the palm plus the volar surface of all digits) is approximately equivalent to 1% of the total body surface area. The degree of pigmentation is estimated to the nearest of one of the following percentages: 100% - complete depigmentation, no pigment is present; 90% - specks of pigment present; 75% - depigmented area exceeds the pigmented area; 50% - pigmented and depigmented areas are equal; 25% - pigmented area exceeds depigmented area; and 10% - only specks of depigmentation present.
The VASI for each body region is determined by the product of the area of vitiligo in hand units and the extent of depigmentation within each hand unit measured patch.
Total body VASI = S All body sites [Hand Units] ´ [Residual depigmentation]
Vitiligo disease activity score (VIDA) The VIDA is a six-point scale for assessing vitiligo activity. Scoring is based on the individual′s own opinion of the present disease activity over time. Active vitiligo involves either expansion of existing lesions or appearance of new lesions. Grading is as follows: VIDA Score +4 - Activity of 6 weeks or less duration; +3 - Activity of 6 weeks to 3 months; +2 - Activity of 3 - 6 months;+1 - Activity of 6 - 12 months; 0 - Stable for 1 year or more; and -1 - Stable with spontaneous repigmentation since 1 year or more. A low VIDA score indicates less activity.
SCLERODERMA
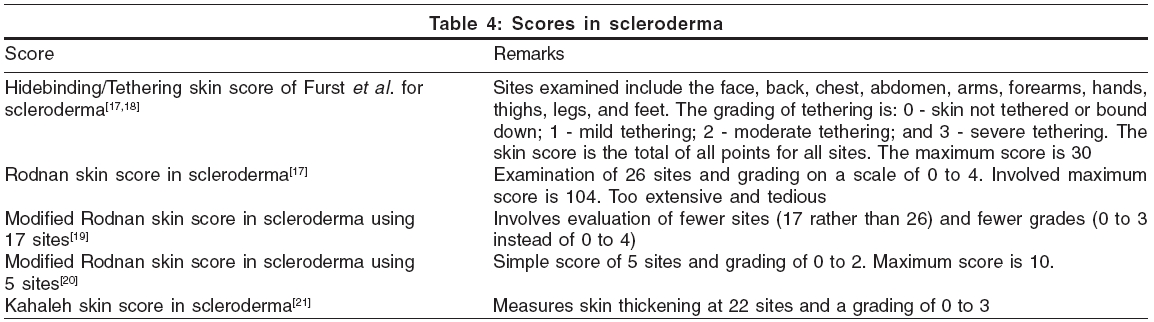
Scores used for scleroderma are summerized in [Table - 4].
HIRSUTISM
The Ferriman and Gallwey score[22] measures hirsutism in women by the degree of hair growth in 11 body regions, out of which the forearm and hand, lower leg and feet are not included in the "hormonal" score. This is a time consuming and apparently complex semiquantitative scoring system for hirsutism. [Table - 5]
Even if hirsutism is present bilaterally on the extremities (upper arms, forearms, thighs and lower legs), only a single value is entered.
Ferriman Gallwey hormonal hair score = Sum of all scores.
The minimum score is zero and the maximum is 36. Obviously, the higher the score, the more hirsute is the woman. A score of less than 8 is considered as non hirsute, 8-16 as mild hirsutism, 17-25 as moderate hirsutism, and more than 25 as severe hirsutism. A score of more than 6 in Caucasian women indicates abnormal hair distribution. Each ethnic group may have a different upper limit of the normal value.
PEMPHIGUS VULGARIS[23]
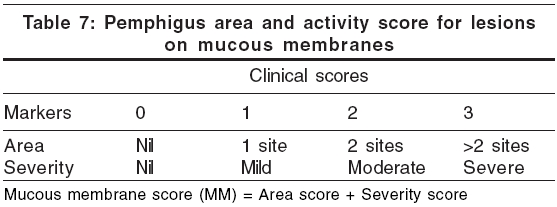
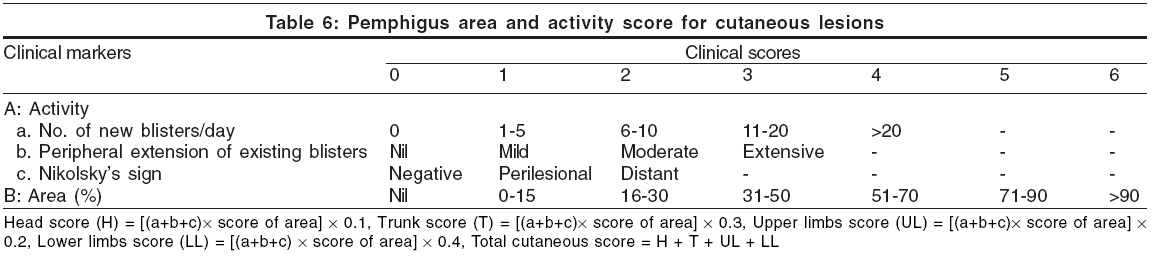
Pemphigus area and activity score (PAAS) is a specific scoring system that has been suggested by Agarwal et al[23] for the clinical assessment of severity and progression of pemphigus vulgaris. PAAS is calculated separately for cutaneous and mucus membrane lesions [Table - 6][Table - 7]. Total score is calculated by adding up the cutaneous score and the mucous membrane score.
MELASMA
Melasma area severity index (MASI) is developed by Kimbrough-Green et al for the assessment of melasma.[24] The severity of the melasma in each of the four regions (forehead, right malar region, left malar region and chin) is assessed based on three variables: percentage of the total area involved (A), darkness (D), and homogeneity (H).
A numerical value assigned for the corresponding percentage area involved is as follows: 0=no involvement; 1=< 10% involvement; 2=10-29% involvement; 3=30-49% involvement; 4=50-69% involvement; 5=70-89% involvement; and 6=90-100% involvement. The darkness of the melasma (D) is compared to the normal skin and graded on a scale of 0 to 4 as follows: 0=normal skin color without evidence of hyperpigmentation; 1=barely visible hyperpigmentation; 2=mild hyperpigmentation; 3=moderate hyperpigmentation; 4=severe hyperpigmentation. Homogeneity of the hyperpigmentation (H) is also graded on a scale of 0 to 4 as follows: 0=normal skin color without evidence of hyperpigmentation; 1=specks of involvement; 2=small patchy areas of involvement < 1.5 cm diameter; 3=patches of involvement> 2 cm diameter; 4=uniform skin involvement without any clear areas).
To calculate the MASI score, the sum of the severity grade for darkness (D) and homogeneity (H) is multiplied by the numerical value of the areas (A) involved and by the percentages of the four facial areas (10-30%).
Total MASI score: Forehead 0.3 (D+H)A + right malar 0.3 (D+H)A + left malar 0.3 (D+H)A + chin 0.1 (D+H)A
ACNE VULGARIS
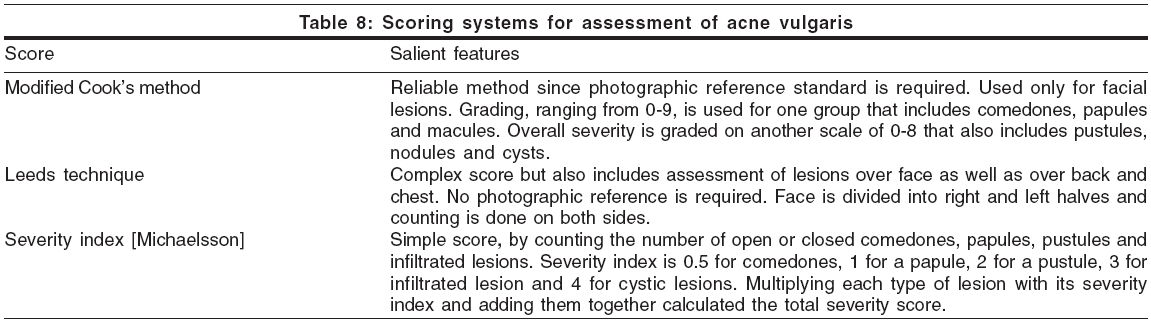
Scoring systems for assessment of acne vulgaris are used in some clinical trials. Salient features of some of these scoring systems like Modified Cook′s method,[25] Leeds technique[26] and severity index described by Michaelsson,[27] are described here [Table - 8].
URTICARIA
Urticaria activity score (UAS) The UAS consisted of the sum of the wheal number score and the itch severity score.[28] The wheal numbers are graded from 0 to 3 as follows: 0 - less than 10 small wheals (diameter, < 3 cm); 1- 10 to 50 small wheals or less than 10 large wheals (diameter, > 3 cm); 2 - greater than 50 small wheals or 10 to 50 large wheals; and 3 - almost the whole body is covered. The severity of the itching is graded from 0 to 3 (0, none; 1, mild; 2, moderate; and 3, severe).
ALOPECIA AREATA
In a study comparing efficacy of azelaic acid and anthralin for patchy alopecia areata, Sansaz et al used terminal hair regrowth score (RGS) which encompasses a scale ranging from 0 (inadequate response) to 2 (complete response).[29]
National Alopecia Areata Foundation working committee has devised "Severity of Alopecia Tool score" (SALT score).[30] Scalp is divided into 4 areas namely, Vertex - 40% (0.4) of scalp surface area; right profile of scalp - 18% (0.18) of scalp surface area; left profile of scalp - 18% (0.18) of scalp surface area; Posterior aspect of scalp - 24% (0.24) of scalp surface area. Percentage of hair loss in any of these areas is percentage hair loss multiplied by percent surface area of the scalp in that area. SALT score is the sum of percentage of hair loss in all above mentioned areas. For e.g., if the percentage hair loss in vertex, right profile, left profile and posterior aspect is 20, 30, 40 and 50% respecively; then, SALT score = (20 ´ 0.4) = (30 ´ 0.18) + (40 ´ 0.18) + (50 ´ 0.24) = 8+5.4+7.2+12 = 32.6
DYSHIDROTIC ECZEMA
Dyshidrotic eczema area and severity index (DASI) is proposed for dyshidrotic eczema.[31]
Dyshidrotic eczema area and severity index (DASI) Based on the severity grade of single items - number of vesicles per square centimetre (V), erythema (E), desquamation (S) and itch (I) - and the extension of the affected area (A) and is calculated with defined score points (p) as: DASI = (pV + pE = pS + pI) ´ pA.
DASI was found to be a simple and useful tool to assess the severity of dyshidrotic eczema and the effect of therapy.
Thus application of mind helps to design scores for semi-objective assessment of skin diseases. Till better objective parameters are developed, scores will continue to remain the gold standard for assessing the severity of dermatological diseases in clinical research.
| 1. |
European Task Force on Atopic Dermatitis. Severity scoring of atopic dermatitis: The SCORAD Index. Dermatology 1993;186:23-31.
[Google Scholar]
|
| 2. |
Jones B. Six area, six sign atopic dermatitis (SASSAD) severity score: A simple system for monitoring disease activity in atopic dermatitis. Br J Dermatol 1996;135:25-30.
[Google Scholar]
|
| 3. |
Van Joost TH, Heule F, Korstanje M, Van den Broek MJ, Stenveld HJ, Van Vloten WA. Cyclosporin in atopic dermatitis: A multicentre placebo-controlled study. Br J Dermatol 1994;130:634-40.
[Google Scholar]
|
| 4. |
Bahmer FA, Schubert HJ. Quantification of the extent and severity of atopic dermatitis: The ADASI score. Arch Dermatol 1991;127:1239-40.
[Google Scholar]
|
| 5. |
Charman C, Williams H. Outcome measures of disease severity in atopic eczema. Arch Dermatol 2000;136:763-9.
[Google Scholar]
|
| 6. |
Costa C, Rilliet A, Nicolet M, Saurat JH. Scoring atopic dermatitis: The simpler the better? Acta Derm Venereol (Stockh) 1989;69:41-5.
[Google Scholar]
|
| 7. |
Van Leent EJ, Grδber M, Thurston M, Wagenaar A, Spuls PI, Bos JD. Effectiveness of the ascomycin macrolactam SDZ ASM 981 in the topical treatment of atopic dermatitis. Arch Dermatol 1998;134:805-9.
[Google Scholar]
|
| 8. |
Charman D, Varigos G, Horne DJ, Oberklaid F. The development of a practical and reliable assessment measure for atopic dermatitis (ADAM). J Outcome Meas 1999;3:21-34.
[Google Scholar]
|
| 9. |
Cherill R, Graeber M, Hanifin J, Omoto M, Thurston M, Tofte S. Eczema area and severity index (EASI): A new tool to evaluate atopic dermatitis [abstract]. J Eur Acad Dermatol Venereol 1998;11:48.
[Google Scholar]
|
| 10. |
Fredriksson T, Pettersson U. Severe psoriasis - Oral therapy with a new retinoid. Dermatologica 1978;157:238-44.
[Google Scholar]
|
| 11. |
Sugai J, Ozawa A, Kawakubo Y, Izuka M, Miyahara M, Okhido M. New method for determining prognosis of patients with psoriasis (E-PAP). J Dermatol Sci 1998;16:165-9.
[Google Scholar]
|
| 12. |
Kirby B, Fortune DG, Bhushan M, Chalmers RJ, Griffiths CE. The Salford Psoriasis Index: An holistic measure of psoriasis. Br J Dermatol 2000;142:728-32.
[Google Scholar]
|
| 13. |
Fleischer AB Jr, Rapp SR, Reboussin DM, Vanarthos JC, Feldman SR. Patient measurement of psoriasis disease severity with a structured instrument. J Invest Dermatol 1994;102:967-9.
[Google Scholar]
|
| 14. |
Bastuji-Garin S, Fouchard N, Bertocchi M, Roujeau JC, Revuz J, Wolkenstein P. SCORTEN: A Serverity-of-Illness Score for Toxic Epidermal Necrolysis. J Invest Derm 2000;115:149-53.
[Google Scholar]
|
| 15. |
Hamzavi I, Jain H, McLean D, Shapiro J, Zeng H, Lui H. Parametric modeling of narrowband UV-B phototherapy for vitiligo using a novel quantitative tool: The Vitiligo Area Scoring Index. Arch Dermatol 2004;140:677-83.
[Google Scholar]
|
| 16. |
Njoo MD, Das PK, Bos JD, Westerhof W. Association of the Koebner phenomenon with disease activity and therapeutic responsiveness in Vitiligo Vulgaris. Arch Dermatol 1999;135:407-13.
[Google Scholar]
|
| 17. |
Pope JE, Baron M, Belamy M, Campbell J, Carette S, Chalmers I, et al . Variability of skin scores and clinical measurements in scleroderma. J Rheumatol 1995;22:1271-6.
[Google Scholar]
|
| 18. |
Clements PJ, Lachenbruch PA, Ng SC, Simmons M, Sterz M, Furst DE. Skin Score. A semiquantitative measure of cutaneous involvement that improves prediction of prognosis in systemic sclerosis. Arthritis Rheum 1990;33:1256-63.
[Google Scholar]
|
| 19. |
Clements P, Lachenbruch P, Seibold J, White G, Weiner S, Martin R, et al . Inter and intraobserver variability of total skin thickness score (modified Rodnan TSS) in systemic sclerosis. J Rheumatol 1995;22:1281-5.
[Google Scholar]
|
| 20. |
Silman A, Harrison M, Brennan P. Is it possible to reduce observer variability in skin score assessment of scleroderma. J Rheumatol 1995;22:1277-80.
[Google Scholar]
|
| 21. |
Kahaleh MB, Sultany GL, Smith EA, Huffstutter JE, Loadholt CB, LeRoy EC. A modified scleroderma skin scoring method. Clin Exp Rheumatol 1986;4:367-9.
[Google Scholar]
|
| 22. |
Ferriman D, Gallwey JD. Clinical assessment of body hair growth in women. J Clin Endocrinol 1961;21:1440-7.
[Google Scholar]
|
| 23. |
Agarwal M, Walia R, Kochar AM, Chander R. Pemphigus Area and Activity Score (PAAS) - A novel clinical scoring method for monitoring of pemphigus vulgaris patients. Int J Dermatol 1998;37:158-60.
[Google Scholar]
|
| 24. |
Kimbrough-Green CK, Griffiths CE, Finkel LJ, Hamilton TA, Bulengo-Ransby SM, Ellis CN, et al . Topical retinoic acid (tretinoin) for melasma in black patients. A vehicle-controlled clinical trial. Arch Dermatol 1994;130:727-33.
[Google Scholar]
|
| 25. |
Cook CH, Centner RL, Michaels SE. An acne grading method using photographic standards. Arch Dermatol 1979;115:571-5.
[Google Scholar]
|
| 26. |
Burke BM, Cunliffe WJ. The assessment of acne vulgaris-The Leeds technique. Br J Dermatol 1984;111:83-92.
[Google Scholar]
|
| 27. |
Michaelsson G, Juhlin L, Vahlquist A. Effect of oral zinc and Vitamin A in acne. Arch Dermatol 1977;113:31-6.
[Google Scholar]
|
| 28. |
Erbagci Z. The leukotriene receptor antagonist montelukast in the treatment of chronic idiopathic urticaria: A single-blind, placebo-controlled, crossover clinical study. J Aller Clin Immunol 2002;110:484-8.
[Google Scholar]
|
| 29. |
Sasmaz S, Arican O. Comparison of azelaic acid and anthralin for the therapy of patchy alopecia areata: A pilot study. Am J Clin Dermatol 2005;6:403-6.
[Google Scholar]
|
| 30. |
Olsen EA, Hordinsky MK, Price VH, Roberts JL, Shapiro J, Canfield D, et al . National Alopecia Areata Foundation. Alopecia areata investigational assessment guidelines. part II. National Alopecia Areata Foundatin. J Am Acad Dermatol 2004;51: 440-7.
[Google Scholar]
|
| 31. |
Vocks E, Plotz SG, Ring J. The Dyshidrotic Eczema Area and Severity Index - A score developed for the assessment of dyshidrotic eczema. Dermatology 1999;198:265-9.
[Google Scholar]
|
Fulltext Views
51,977
PDF downloads
10,600





