Translate this page into:
Associations between interleukin-13, interleukin-4 and their receptor gene polymorphisms and susceptibility to atopic dermatitis in a Chinese Han population
Corresponding author: Prof. Lian-Sheng Zhong, Department of Dermatology, Xiamen Children’s Hospital (Children’s Hospital of Fudan University Xiamen Branch), Xiamen, Fujian, China. 248694437@qq.com
-
Received: ,
Accepted: ,
How to cite this article: Zhong LS, Chen XY, Xiao J. Associations between interleukin-13, interleukin-4 and their receptor gene polymorphisms and susceptibility to atopic dermatitis in a Chinese Han population. Indian J Dermatol Venereol Leprol. 2024;90:769-76. doi: 10.25259/IJDVL_470_2023
Abstract
Background
Atopic dermatitis (AD) is a common skin condition that occurs due to a combined effect of immune dysregulation, skin barrier dysfunction, changes in the cutaneous microbiome, and genetic factors. Recent data from both clinical trials and real-world studies indicate that dupilumab, a biological agent that inhibits interleukin 4 receptor-α is an effective drug in the treatment of AD, which further suggests the important role of IL-13 and IL-4 in the pathogenesis of AD.
Objectives
To assess the association between gene polymorphisms of IL-13, IL-13 receptor, IL-4, and IL-4 receptor and susceptibility to AD.
Methods
The single nucleotide polymorphisms (SNPs) of the above-mentioned genes were detected by single base extension (SNaPshot) assay. The association between these SNPs and AD risk was analysed using SPSS software.
Results
Two hundred and seventy-one subjects including 130 patients with AD and 141 healthy controls were enrolled. There were statistical differences between AD patients and controls in genotype distribution at rs2265753, rs6646259, and rs2254672 of the IL-13 receptor gene (P all < 0.001). Subjects with CG at rs2265753, AG at rs6646259 and TG at rs2254672 had increased risks for AD (P all < 0.001), and subjects with GG at rs2265753, rs6646259, and rs2254672 had reduced risks for AD (P all < 0.001).
Limitation
This was a single-centre and single-race study, with a relatively small sample size.
Conclusions
Findings from this study show that rs2265753, rs6646259 and rs2254672 of the IL-13 receptor gene are associated with susceptibility to AD.
Keywords
Atopic dermatitis
IL-14
IL-13
single nucleotide polymorphism
receptor
Introduction
Atopic dermatitis (AD) is a common skin disease that usually begins during infancy. The lesions of AD are characterised by pruritic erythematous, papules, papulovesicles, and lichenification which may become excoriated and tend to have a flexural distribution such as the neck, cubital fossa, and popliteal fossa. AD generally occurs due to a combined effect of immune dysregulation, skin barrier dysfunction, alterations in the skin bacterial microbiome, and genetic factors.1 Genetic factors are very important in the pathogenesis of AD, which is supported by the facts that the concordance rate for AD is much higher in identical twins than in fraternal twins,2 AD patients or their first-degree relatives often have a history of atopic diseases, such as AD, allergic rhinitis, allergic conjunctivitis, asthma, food allergy, and eosinophilic esophagitis. Recent studies have shown that more than 70 genes may be related to the pathogenesis of AD.3
Recent data from both clinical trials and real-world studies indicate that dupilumab, a biological agent targeting interleukin 4 receptor-α is a very effective drug for the treatment of AD, emphasising the role of IL-13 and IL-4 in the pathogenesis.4 The objective of this study was to evaluate the association between IL-13, IL-4, and their receptor gene polymorphisms and the susceptibility to AD in a Chinese Han population.
Methods
Study population
The required sample size was calculated using the Raosoft sample size calculator,5 based on a significance level of 5%, confidence interval of 90%, population size of 2,00,000, and the prevalence of each mutation [Table 1]. Inclusion criteria were a confirmed diagnosis of AD based on Hanifin and Rajka criteria, absence of other skin diseases, and Chinese Han ethnicity. Patients with mixed ethnicities were excluded. All patients were enrolled from October 2021 to July 2022 in Xiamen Children’s Hospital. Healthy controls were enrolled from children undergoing a routine physical examination in the same hospital, and all of them had no personal and/or family history of AD, allergic rhinitis, asthma, and other allergic diseases based on the questionnaire and previous diagnoses. The study was approved by the Ethics Committee of Xiamen Children’s Hospital (Approval no. [2022]04) and all patients and controls signed informed consent before data collection.
| SNP | Minor allele | Minor allele frequency | Sample size required |
|---|---|---|---|
| rs30913076 | G | 0.213 | 182 |
| rs22546726 | G | 0.407 | 261 |
| rs22657536 | G | 0.408 | 262 |
| rs205416 | A | 0.360 | 250 |
| rs18009257 | T | 0.215 | 183 |
| rs22432508 | T | 0.165 | 149 |
| rs22272848 | T | 0.341 | 243 |
| rs18050118 | C | 0.173 | 155 |
| rs18012759 | G | 0.188 | 166 |
| rs18050109 | G | 0.500 | 271 |
| rs6646259 | A | 0.500* | 271 |
| rs2243274 | G | 0.500* | 271 |
Primer design, data collection, and genotyping
Blood was collected from each patient and control and immediately stored at –80°C. We searched the website of The National Center for Biotechnology Information (NCBI) to identify Single Nucleotide Polymorphisms (SNPs) in IL-13, IL-4, and their receptor genes, reviewed relevant literature, and in total choose 12 SNPs from the four genes as listed, IL-13 -rs3091307, rs2054, and rs1800925, IL-13 receptor - rs2265753, rs6646259 and rs2254672, IL-4 - rs2243250, rs2227284 and rs2243274 and IL-4 receptor - rs1801275, rs1805010 and rs1805011. Genotypes of samples were detected by SNaPshot assay, polymerase chain reaction (PCR) primers used for amplification, and minisequencing primers used for SNaPshot reactions of the 12 gene regions as shown in Table 2.
| PCR primers used for the amplification of the 12 gene regions | |||
|---|---|---|---|
| Genes | SNPs | Primer sequence | Product (bp) |
| IL-4 | rs2243250 |
F: AAGGGCTTCCTTATGGGTAAGG R:GCATCTTGGAAACTGTCCTGTC |
208 |
| rs2227284 | F:CTGTCTGAGGAACAGCAAAGTG R:GAACTGCTTAGGGAGTGACTCA | 386 | |
| rs2243274 |
F:AAGGAGATTCTCACTCCGCATC R:TCTCAGTCAGGTTCTGCTCTTG |
345 | |
| IL-13 | rs3091307 |
F:AGATACAGAGGTGTTATAGTG R:AGTTCCTGAGCATTCTTG |
343 |
| rs20541 |
F:CTTCCGTGAGGACTGAATGAGA R:CACAGGCTGAGGTCTAAGCTAA |
360 | |
| rs1800925 |
F:TGGGTAGGGGAGAAATCTTGAC R:ACGTGTCTGGCCCCTTTAAT |
360 | |
| IL-4R | rs1805010 |
F:ATCTGTCCTCACATCCGTGATC R:CTTCCTCCTGCTGTTGCTATGA |
393 |
| rs1805011 |
F:AGATCAGCAAGACAGTCCTCTG R:AGGAACAGGCTCTCTGTTAGC |
202 | |
| rs1801275 |
F:AGAGTCCAGACAACCTGACTTG R:CTTGAGAAGGCCTTGTAACCAG |
394 | |
| IL-13R | rs2265753 |
F:CATTTGAGGAGAGACTCCCAGT R:CCAAGTCAGTTCTTCACTCAGC |
329 |
| rs6646259 |
F:AGCCTGGACCTCTATTACTCCT R:TCGTTGAAGAGGCTGTTGGT |
356 | |
| rs2254672 |
F:GTCATCATTCCCTTCGACAGC R:GGCCTAGCACAAACCAAAGAC |
257 | |
| Minisequencing primers used for the SNaPshot reactions of the 12 gene regions | ||
| Genes | SNPs | Primer sequence |
| IL-4 | rs2243250 | TTTTTTTTTTTTTTTTTTTTTTTTTTCACCTAAACTTGGGAGAACATTGT |
| rs2227284 | TTTTTTTTTTTAGCTCTCTTTGGTAAATAGGAAAT | |
| rs2243274 | TTTTTTTTTTTTTTTTAAAATGTCTTAGCTCCTCACTTGG | |
| IL-13 | rs3091307 | TTTTTTTTTTTTTTTTTTTTGTTTGTGTTTATTCCATTGTTTTCA |
| rs20541 | TTTTTTTTTTTTTTTTTTTTTTTTTTTTGCTTTCGAAGTTTCAGTTGAAC | |
| rs1800925 | TTTTTTTTTTTTTTTTTTTTCCTTTTCCTGCTCTTCCCTC | |
| IL-4R | rs1805010 | TTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTCCTCCGTTGTTCTCAGGGA |
| rs1805011 | TTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTGACTTCCAGGAGGGAAGGG | |
| rs1801275 | TTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTCCCCCACCAGTGGCTATC | |
| IL-13R | rs2265753 | TTTTTTTTTTTTTTTTTTTTTTTTTTTTGCCATGGCCTGCGTGAT |
| rs6646259 | TTTTTTTTTTTTTTTTTTTTTTTTTTTTTTGTTGTCGCGGACACTCCATA | |
| rs2254672 | TTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTCCACTGCCCCTAACAGCCTA | |
Note: A-adenine, C-cytosine, T-thymine and G-guanine.
Statistical analysis
SPSS 21.0 software was applied to analyse all the experimental data. The age of the patients and controls were analysed by Student’s t-test. The gender of the two groups and the deviation of Hardy–Weinberg equilibrium (HWE) were analysed by the chi-square test. Differences in allelic frequency and genotypic distribution including dominant, codominant, over dominant, and recessive genetic models between AD patients and healthy controls were evaluated using logistic regression analyses, and the odd ratios (OR) with the 95% confidence interval (95% CI) were obtained accordingly.
Results
We enrolled 130 patients with AD (64 males and 66 females, mean age 39.74 ± 31.49 months) and 141 healthy controls (72 males and 69 females, mean age 41.45 ± 29.68 months) less than 18 years of age. No significant differences were observed concerning gender and age between patients and controls (P > 0.05). All 271 samples were successfully genotyped for the selected 12 SNPs, and they did not deviate from the distribution of Hardy–Weinberg equilibrium [Table 3]. Representative electropherograms of the 12 SNPs are shown in Figure 1. Table 3 lists the minor allele frequency of the 12 SNPs with a 95% CI level. Table 4 lists the allele and genotype frequencies of the 12 SNPs in the AD patient group and the control group. There were no statistically significant differences between AD patients and controls in genotype and allele frequencies at SNPs of IL-13, IL-4, and IL-4 receptor genes. There were no statistically significant differences between AD patients and controls in allele frequencies at SNPs of the IL-13 receptor gene. There were statistically significant differences between AD patients and controls in genotype distribution at rs2265753, rs6646259, and rs2254672 of the IL-13 receptor gene (P all < 0.001). Subjects with CG at rs2265753, AG at rs6646259, and TG at rs2254672 had increased risks for AD (P all < 0.001), and subjects with GG at rs2265753, rs6646259, and rs2254672 had reduced risks for AD (P all < 0.001).
| Hardy–Weinberg equilibrium test | Minor allele frequency with 95% CI | |||||||
|---|---|---|---|---|---|---|---|---|
| SNPs | Genotype distribution | χ2 | P | Minor allele | Minor allele frequency | 95% CI | ||
| rs2265753 | CC | CG | GG | 4.08 | 0.13 | G | 0.446 | 0.385–0.507 |
| 48 | 48 | 34 | ||||||
| rs6646259 | AA | AG | GG | 3.19 | 0.20 | G | 0.419 | 0.359–0.480 |
| 51 | 49 | 30 | ||||||
| rs2254672 | TT | TG | GG | 4.97 | 0.08 | G | 0.423 | 0.363–0.484 |
| 52 | 46 | 32 | ||||||
| rs3091307 | AA | AG | GG | 2.94 | 0.23 | G | 0.219 | 0.169–0.270 |
| 75 | 53 | 2 | ||||||
| rs20541 | GG | AG | AA | 1.19 | 0.55 | A | 0.362 | 0.303–0.420 |
| 49 | 68 | 13 | ||||||
| rs1800925 | CC | CT | TT | 0.63 | 0.73 | T | 0.188 | 0.141–0.236 |
| 84 | 43 | 3 | ||||||
| rs2243250 | TT | CT | CC | 0.01 | 1.00 | C | 0.185 | 0.137–0.232 |
| 86 | 40 | 4 | ||||||
| rs2227284 | TT | GT | GG | 0.27 | 0.87 | G | 0.131 | 0.090–0.172 |
| 99 | 28 | 3 | ||||||
| rs2243274 | AA | AG | GG | 0.00 | 1.00 | G | 0.192 | 0.144–0.241 |
| 85 | 40 | 5 | ||||||
| rs1801275 | AA | GA | GG | 0.24 | 0.89 | G | 0.169 | 0.123–0.215 |
| 91 | 34 | 5 | ||||||
| rs1805010 | GG | GA | AA | 0.88 | 0.65 | A | 0.477 | 0.416–0.538 |
| 32 | 72 | 26 | ||||||
| rs1805011 | AA | CA | CC | 0.25 | 0.99 | C | 0.073 | 0.041–0.105 |
| 112 | 17 | 1 | ||||||
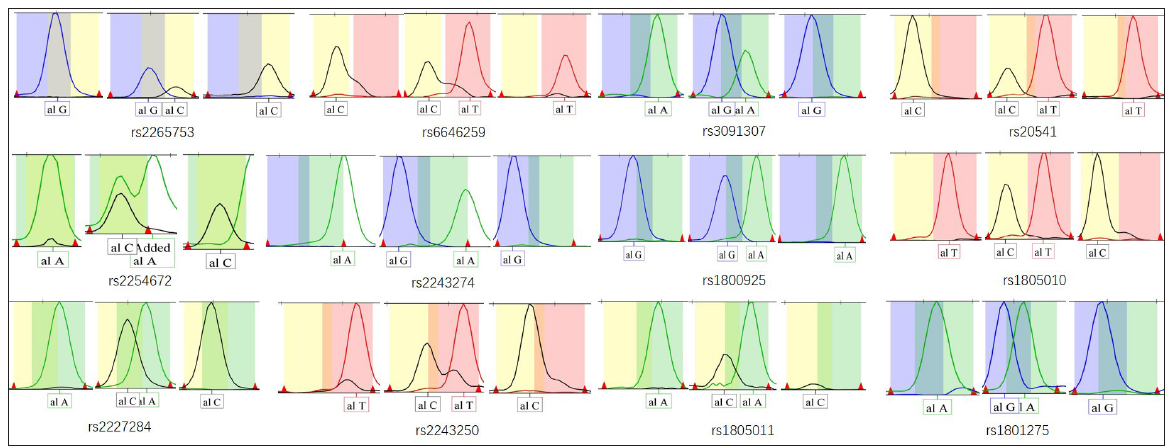
- Representative electropherograms of the 12 Single Nucleotide Polymorphisms (SNPs). Peaks of different colors indicates different alleles (Blue peak: G; Black peak: C; Green peak: A; Red peak: T). The overlapping peaks at rs 2254672 means alleles C and A at the same site but in different color.
| SNP | Genetic model | Genotype/allele | Patients N = 130(%) | Controls N = 141(%) | Logistic regression | |
|---|---|---|---|---|---|---|
| OR (95% CI) | P value | |||||
| IL-13R | ||||||
| rs2265753 | Codominant | CC | 48 (36.9) | 62 (44) | 1 | <0.001 |
| CG | 48 (36.9) | 14 (9.9) | 0.226 (0.112–0.457)) | |||
| GG | 34 (26.2) | 65 (46.1) | 1.480 (0.845–2.592) | |||
| Dominant | CC | 48 (36.9) | 62 (44) | 1 | 0.238 | |
| CG+GG | 82 (63.1) | 79 (65.1) | 0.746(0.458–1.214) | |||
| Recessive | CC+CG | 96 (73.8) | 76 (53.9) | 1 | 0.001 | |
| GG | 34 (26.2) | 65 (46.1) | 2.415 (1.447–4.031) | |||
| Over dominant | CC+GG | 82 (63.1) | 127 (90.1) | 1 | <0.001 | |
| CG | 48 (36.9) | 14 (9.9) | 0.188 (0.098–0.363) | |||
| Alleles | C | 144 (55.4) | 138 (48.9) | 1 | 0.133 | |
| G | 116 (44.6) | 144 (51.1) | 1.295 (0.924–1.186) | |||
| rs6646259 | Codominant | AA | 51 (39.2) | 66 (48.8) | 1 | <0.001 |
| AG | 49 (37.7) | 13 (9.2) | 0.205 (0.101–0.418) | |||
| GG | 30 (23.1) | 62 (44.0) | 1.597 (0.904–2.820) | |||
| Dominant | AA | 51 (39.2) | 66 (48.8) | 1 | 0.208 | |
| AG+GG | 79 (60.8) | 75 (53.2) | 0.734 (0.453–1.189) | |||
| Recessive | AA+AG | 100 (76.9) | 79 (56.0) | 1 | <0.001 | |
| GG | 30 (23.1) | 62 (44.0) | 2.616 (1.545–4.428) | |||
| Over dominant | AA+GG | 81 (62.3) | 128 (90.1) | 1 | <0.001 | |
| AG | 49 (37.7) | 13 (9.1) | 0.168 (0.086–0.329) | |||
| Alleles | A | 151 (58.1) | 145 (51.4) | 1 | 0.120 | |
| G | 109 (41.9) | 137 (48.6) | 1.309 (0.932–1.838) | |||
| rs2254672 | Codominant | TT | 52 (40) | 63 (44.7) | 1 | <0.001 |
| TG | 46 (35.4) | 13 (9.2) | 0.233 (0.114–0.478) | |||
| GG | 32 (24.6) | 65 (46.1) | 1.677 (0.957–2.936) | |||
| Dominant | TT | 52 (40) | 63 (44.7) | 1 | 0.436 | |
| TG+GG | 78 (60.0) | 78 (55.3) | 0.825 (0.509–1.338) | |||
| Recessive | TT+TG | 98 (75.4) | 76 (57.6) | 1 | <0.001 | |
| GG | 32 (24.6) | 65 (42.4) | 2.619 (1.559-–4.339) | |||
| Over dominant | TT+GG | 84 (64.6) | 128 (90.8) | 1 | <0.001 | |
| TG | 46 (35.4) | 13 (9.2) | 0.185 (0.094–0.364) | |||
| Alleles | T | 150 (57.7) | 139 (49.3) | 1 | 0.050 | |
| G | 110 (42.3) | 143 (50.7) | 1.403 (0.999–1.969) | |||
| IL-13 | ||||||
| rs3091307 | Codominant | AA | 75 (57.7) | 91 (64.5) | 1 | 0.139 |
| AG | 53 (40.8) | 44 (31.2) | 0.684 (0.414–1.132) | |||
| GG | 2 (1.5) | 6 (4.3) | 2.473 (0.485–12.610) | |||
| Dominant | AA | 75 (57.7) | 91 (64.5) | 1 | 0.248 | |
| AG+GG | 55 (42.3) | 50 (35.5) | 0.749 (0.459–1.223) | |||
| Recessive | AA+AG | 128 (98.5) | 135 (95.7) | 1 | 0.176 | |
| GG | 2 (1.5) | 6 (4.3) | 2.844 (0.564–14.351) | |||
| Over dominant | AA+GG | 77 (59.2) | 97 (68.8) | 1 | 0.101 | |
| AG | 53 (40.8) | 44 (31.2) | 0.659 (0.400–1.086) | |||
| Alleles | A | 203 (78.1) | 226 (80.1) | 1 | 0.555 | |
| G | 57 (21.9) | 56 (19.9) | 0.882 (0.583–1.336) | |||
| rs20541 | Codominant | GG | 49 (37.7) | 64 (45.4) | 1 | 0.434 |
| AG | 68 (52.3) | 64 (45.4) | 0.721 (0.435–1.194) | |||
| AA | 13 (10.0) | 13 (9.2) | 0766 (0.326–1.799) | |||
| Dominant | GG | 49 (37.7) | 64 (45.4) | 1 | 0.199 | |
| AG+AA | 81 (62.3) | 77 (54.6) | 0.728 (0.448–1.183) | |||
| Recessive | GG+AG | 117 (90.0) | 128 (90.8) | 1 | 0.828 | |
| AA | 13 (10.0) | 13 (9.2) | 0.914 (0.407–2.052) | |||
| Over dominant | AA+GG | 62 (47.7) | 77 (54.6) | 1 | 0.255 | |
| AG | 68 (52.3) | 64 (45.4) | 0.758 (0.470–1.222) | |||
| Alleles | A | 94 (36.2) | 90 (31.9) | 1 | 0.298 | |
| G | 166 (63.8) | 192 (68.1) | 1.208 (0.846–1.725) | |||
| rs1800925 | Codominant | CC | 84 (64.6) | 94 (66.7) | 1 | 0.938 |
| CT | 43 (33.1) | 44 (31.2) | 0.914 (0.547–1.527) | |||
| TT | 3 (2.3) | 3 (2.1) | 0.894 (0.176–4.548) | |||
| Dominant | CC | 84 (64.6) | 94 (66.7) | 1 | 0.722 | |
| CT+TT | 46 (35.4) | 47 (33.3) | 0.913 (0.553–1.508) | |||
| Recessive | CC+CT | 127 (97.7) | 138 (97.9) | 1 | 0.920 | |
| TT | 3 (2.3) | 3 (2.1) | 0.920 (0.182–4.643) | |||
| Over dominant | CC+TT | 87 (66.9) | 97 (68.8) | 1 | 0.742 | |
| CT | 43 (33.1) | 44 (31.2) | 0.918 (0.551–1.529) | |||
| aAlleles | C | 211 (81.2) | 232 (82.3) | 1 | 0.737 | |
| T | 49 (18.8) | 50 (17.7) | 0.928 (0.600–1.435) | |||
| IL-4 | ||||||
| rs2243250 | Codominant | TT | 86 (66.1) | 91 (64.5) | 1 | 0.818 |
| CT | 40 (30.8) | 45 (31.9) | 1.063 (0.633–1.785) | |||
| CC | 4 (3.1) | 5 (3.6) | 1.575 (0.365–6.792) | |||
| Dominant | TT | 86 (66.1) | 91 (64.5) | 1 | 0.780 | |
| CT+CC | 44 (33.9) | 50 (35.5) | 1.074 (0.651–1.772) | |||
| Recessive | TT+CT | 126 (96.9) | 136 (96.4) | 1 | 0.830 | |
| CC | 4 (3.1) | 5 (3.6) | 1.158 (0.304–4.409) | |||
| Over dominant | CC+TT | 90 (69.2) | 96 (68.1) | 1 | 0.839 | |
| CT | 40 (30.8) | 45 (31.9) | 1.055 (0.631–1.763) | |||
| Alleles | C | 48 (18.5) | 55 (19.5) | 1 | 0.757 | |
| T | 212 (81.5) | 227 (80.5) | 0.934 (0.608–1.437) | |||
| rs2227284 | Codominant | TT | 99 (76.2) | 115 (81.6) | 1 | 0.382 |
| GT | 28 (21.5) | 25 (17.7) | 0.769 (0.421–1.404) | |||
| GG | 3 (2.3) | 1 (0.7) | 0.287 (0.029–2.803) | |||
| Dominant | TT | 99 (76.2) | 115 (81.6) | 1 | 0.275 | |
| GT+GG | 31 (23.8) | 26 (18.4) | 0.722 (0.402–1.298) | |||
| Recessive | GG | 3 (2.3) | 1 (0.7) | 1 | 0.276 | |
| GT+TT | 127 (97.7) | 140 (99.3) | 3.307 (0.340–32.199) | |||
| Over dominant | GG+TT | 102 (78.5) | 116 (82.3) | 1 | 0.430 | |
| GT | 28 (21.5) | 25 (17.7) | 0.785 (0.430–1.432) | |||
| Alleles | T | 226 (86.9) | 255 (90.4) | 1 | 0.197 | |
| G | 34 (13.1) | 27 (9.6) | 0.704 (0.412–1.203) | |||
| rs2243274 | Codominant | AA | 85 (65.4) | 89 (63.1) | 1 | 0.872 |
| AG | 40 (30.8) | 45 (31.9) | 1.074 (0.639–1.806) | |||
| GG | 5 (3.8) | 7 (5.0) | 1.337 (0.409–4.375) | |||
| Dominant | AA | 85 (65.4) | 89 (63.1) | 1 | 0.698 | |
| AG+GG | 45 (34.6) | 52 (39.9) | 1.104 (0.671–1.815) | |||
| Recessive | GG | 5 (3.8) | 7 (5.0) | 1 | 0.655 | |
| AG+AA | 125 (96.2) | 134 (95.0) | 0.766 (0.237–2.475) | |||
| Over dominant | AA+GG | 90 (69.2) | 96 (68.1) | 1 | 0.568 | |
| AG | 40 (30.8) | 45 (31.9) | 1.160 (0.697–1.932) | |||
| Alleles | A | 210 (80.8) | 223 (79.1) | 1 | 0.624 | |
| G | 50 (19.2) | 59 (20.9) | 1.111 (0.729–1.693) | |||
| IL-4R | ||||||
| rs1801275 | Codominant | AA | 91 (70.0) | 103 (73.1) | 1 | 0.440 |
| GA | 34 (26.2) | 36 (25.5) | 0.935 (0.541–1.617) | |||
| GG | 5 (3.8) | 2 (1.4) | 0.353 (0.067–1.866) | |||
| Dominant | AA | 91 (70.0) | 103 (73.1) | 1 | 0.578 | |
| GA+GG | 39 (30) | 38 (26.9) | 0.861 (0.508–1.460) | |||
| Recessive | GG | 5 (3.8) | 2 (1.4) | 1 | 0.208 | |
| GA+AA | 125 (96.2) | 139 (98.6) | 2.780 (0.530–14.585) | |||
| Over dominant | AA+GG | 96 (73.8) | 105 (74.5) | 1 | 0.907 | |
| GA | 34 (26.2) | 36 (25.5) | 0.968 (0.562–1.668) | |||
| Alleles | A | 216 (83.1) | 242 (85.8) | 1 | 0.379 | |
| G | 44 (16.9) | 40 (14.2) | 0.811 (0.509–1.293) | |||
| rs1805010 | Codominant | GG | 32 (24.6) | 33 (23.4) | 1 | 0.473 |
| GA | 72 (55.4) | 71 (50.4) | 0.956 (0.532–1.719) | |||
| AA | 26 (20.0) | 37 (26.2) | 1.380 (0.686–2.775) | |||
| Dominant | GG | 32 (24.6) | 33 (23.4) | 1 | 0.816 | |
| GA+AA | 98 (75.4) | 108 (76.6) | 1.069(0.612–1.867) | |||
| Recessive | AA | 26 (20.0) | 37 (26.2) | 1 | 0.224 | |
| GA+GG | 104 (80.0) | 104 (73.8) | 0.703 (0.397–1.243) | |||
| Over dominant | GG+AA | 58 (44.6) | 70 (49.6) | 1 | 0.407 | |
| GA | 72 (55.4) | 71 (50.4) | 0.817 (0.507–1.318) | |||
| Alleles | G | 136 (52.3) | 137 (48.6) | 1 | 0.386 | |
| A | 124 (47.7) | 145 (51.4) | 1.161 (0.828–1.626) | |||
| rs1805011 | Codominant | AA | 112 (86.2) | 120 (85.8) | 1 | 0.964 |
| CA | 17 (13.1) | 20 (14.2) | 1.098 (0.547–2.202) | |||
| CC | 1 (0.7) | 1 (0.0) | 0.933 (0.058–15.101) | |||
| Dominant | AA | 112 (86.2) | 120 (85.8) | 1 | 0.806 | |
| CA+CC | 18 (13.8) | 21 (14.2) | 1.089 (0.552–2.150) | |||
| Recessive | CC | 1 (0.7) | 1 (0.0) | 1 | 0.954 | |
| CA+AA | 129 (99.3) | 140 (100.0) | 1.085 (0.067–17.530) | |||
| Over dominant | AA+CC | 113 (86.9) | 121 (85.8) | 1 | 0.791 | |
| CA | 17 (13.1) | 20 (14.2) | 1.099 (0.548–2.203) | |||
| Alleles | A | 241 (92.7) | 260 (92.9) | 1 | 0.828 | |
| C | 19 (7.3) | 22 (7.1) | 1.073 (0.567–2.032) | |||
Discussion
As most patients with AD have eosinophilia and elevated serum total IgE, AD is currently considered to be a Th2 cells-mediated inflammatory disease. In the Th2 inflammatory process of AD, Th2 cells-derived cytokines, especially IL-4 and IL-13 play a crucial role because both can stimulate eosinophil recruitment and IgE production, and contribute to decreased antimicrobial peptide production, increase keratinocyte proliferation and impairment of skin barrier function.10
In most patients with AD, the serum total IgE level is significantly elevated. Therefore, IL-4 has long been considered the most critical cytokine involved in the pathogenesis of AD, because it plays a crucial role in the regulation of IgE synthesis. However, in recent years, strong evidence has shown that IL-13 is more important than IL-4 in AD inflammation.11–13
In recent years, advances in biologics also suggest that IL-13 plays a more important role than IL-4 in the pathogenesis of AD. As mentioned above, clinical and real-world data indicate that dupilumab which blocks the biological functions of both IL-4 and IL-13 has shown very good efficacy in the treatment of AD.4 Lebrikizumab and tralokinumab which blocks the biological function of IL-13 alone, have also shown promising efficacy in the treatment of AD,14,15 and tralokinumab has been approved by FDA for the treatment of moderate to severe AD in 2022.16 However, till date there is no evidence to prove that a biological agent that exclusively blocks IL-4 is effective in the treatment of AD.
In recent years, studies have increasingly focused on the relationship between genetic factors and susceptibility to AD. These studies have shown that more than 70 genes could be involved in the pathogenesis of AD, especially the filaggrin gene.3 Because IL-13 plays a crucial role in the pathogenesis of AD, the polymorphism of IL-13 and its receptor genes could be associated with the genetic susceptibility and severity of AD. In recent years, many researchers have shown that the SNPs of the IL-13 gene, such as rs20541, rs1800925, rs3091307, and rs1295685 are associated with AD. The association between rs20541 and AD susceptibility and total serum IgE level in a German population was reported by Liu et al.17 Lee et al. reported that rs20541 and rs1295685 of the IL-13 gene showed significant associations with AD risk and the total serum IgE level.7 The frequency of allele and genotypes of rs1800925 was found to be associated with the incidence and severity of AD, and total serum IgE level in Polish patients in the research of Gleń et al.18 In a research by Namkung et al., there were significant differences in the genotypic and allelic distributions of rs20541, rs3091307, rs2254672 and rs2265753 between AD patients and normal controls in a Korean population.6 In recent years, many studies have also shown that gene polymorphisms of IL-4 and IL-4 receptor are also related to AD susceptibilities, such as rs2243250, rs2243248, rs2243274 and rs2227284 of IL-4,19–21 and rs1805015, rs1805010, rs1805011 and rs1801275 of IL-4R.22–25
Limitations
This was a single-centre and single-race study with a small sample size. We did not apply the Bonferroni correction in the present study to control the multiple comparisons.
Conclusion
This study researched the association between the polymorphism of IL-4 and IL-13, and their receptor genes, and the genetic susceptibility of AD patients in a Chinese Han Population. The results suggested that the rs2265753, rs6646259, and rs2254672 of the IL-13 receptor gene are associated with susceptibility to AD. Subjects with CG at rs2265753, AG at rs6646259, and TG at rs2254672 had increased risks for AD, while patients with GG at rs2265753, rs6646259 and rs2254672 showed a protective effect. These findings may have a role in the development of future precision therapy and targeted drugs in the treatment of AD. Other functional SNPs of the IL-13 receptor gene need to be explored. Large-scale, multi-centric studies including patients from different ethnicities are required to further clarify the role of the IL-13 receptor gene in the pathogenesis of AD.
Acknowledgements
Nil.
Ethical approval
This research/study was approved by the Institutional Review Board at the Ethics Committee of Xiamen Children’s Hospital, number [2022]04, dated 12 May 2021.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
References
- Importance of genetic factors in the etiology of atopic dermatitis: A twin study. Allergy Asthma Proc. 2007;28:535-9.
- [CrossRef] [PubMed] [Google Scholar]
- Genetic and epigenetic aspects of atopic dermatitis. Int J Mol Sci. 2020;21:6484.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- The efficacy and safety of dupilumab in Chinese patients with moderate-to-severe atopic dermatitis: A randomized, double-blind, placebo-controlled study. Br J Dermatol. 2022;186:633-41.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Available from: http://www.raosoft.com/samplesize.html
- Association of polymorphisms in genes encoding IL-4, IL-13 and their receptors with atopic dermatitis in a Korean population. Exp Dermatol. 2011;20:915-19.
- [CrossRef] [PubMed] [Google Scholar]
- Association of IL13 genetic polymorphisms with atopic dermatitis: Fine mapping and haplotype analysis. Ann Allergy Asthma Immunol. 2020;125:287-93.
- [CrossRef] [PubMed] [Google Scholar]
- Polymorphisms in the interleukin 4, interleukin 4 receptor and interleukin 13 genes and allergic phenotype: A case control study. Adv Med Sci. 2016;61:40-5.
- [CrossRef] [PubMed] [Google Scholar]
- Interaction between antibiotic use and MS4A2 gene polymorphism on childhood eczema: A prospective birth cohort study. BMC Pediatr. 2021;14:314.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- An integrated model of atopic dermatitis biomarkers highlights the systemic nature of the disease. J Invest Dermatol. 2017;137:603-13.
- [CrossRef] [PubMed] [Google Scholar]
- Cytokine profiles in interstitial fluid from chronic atopic dermatitis skin. J Eur Acad Dermatol Venereol. 2015;29:2136-44.
- [CrossRef] [PubMed] [Google Scholar]
- Relative importance of IL-4 and IL-13 in lesional skin of atopic dermatitis. Arch Dermatol Res. 2004;295:459-64.
- [CrossRef] [PubMed] [Google Scholar]
- Atopic dermatitis is an IL-13-dominant disease with greater molecular heterogeneity compared to psoriasis. J Invest Dermatol. 2019;139:1480-9.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Efficacy and safety of lebrikizumab, a high-affinity interleukin 13 inhibitor, in adults with moderate to severe atopic dermatitis: A phase 2b randomized clinical trial. JAMA Dermatol. 2020;156:411-20.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Tralokinumab plus topical corticosteroids for the treatment of moderate-to -severe atopic dermatitis: Results from the double-blind, randomized, multicentre, placebo-controlled phase III ECZTRA 3 trial. Br J Dermatol. 2021;184:450-63.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- The efficacy and safety of IL-13 inhibitors in atopic dermatitis: A systematic review and meta-analysis. Front Immunol. 2022;13:923362.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- An IL13 coding region variant is associated with a high total serum IgE level and atopic dermatitis in the German multicenter atopy study (MAS-90) J Allergy Clin Immunol. 2000;106:167-70.
- [CrossRef] [PubMed] [Google Scholar]
- Interleukin-13 promoter gene polymorphism -1112 C/T is associated with atopic dermatitis in Polish patients. Acta Dermatovenerol Croat. 2012;20:231-8.
- [PubMed] [Google Scholar]
- IL-4 gene polymorphism may contribute to an increased risk of atopic dermatitis in children. Dis Markers. 2016;2016:1021942.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Interleukin 4 -590C/T (rs2243250) polymorphism is associated with increased risk of atopic dermatitis: Meta-analysis of case-control studies. Dermatitis. 2017;28:144-151.
- [CrossRef] [PubMed] [Google Scholar]
- Gene polymorphisms of 22 cytokines in Macedonian children with atopic dermatitis. Iran J Allergy Asthma Immunol. 2012;11:37-50.
- [PubMed] [Google Scholar]
- Gene polymorphism of interleukin-4, interleukin-4 receptor and STAT6 in children with atopic dermatitis in Taif, Saudi Arabia. Immunol Invest. 2016;45:223-34.
- [CrossRef] [PubMed] [Google Scholar]
- Association analysis of non-synonymous polymorphisms of interleukin-4 receptor-α and interleukin-13 genes in canine atopic dermatitis. J Vet Med Sci. 2020;82:1253-9.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Interleukin 4 receptor alpha chain polymorphism Gln551Arg is associated with adult atopic dermatitis in Japan. Br J Dermatol. 2000;142:1003-6.
- [CrossRef] [PubMed] [Google Scholar]
- Case-control study of eczema in relation to IL4Rα genetic polymorphisms in Japanese women: The Kyushu Okinawa maternal and child health study. Scand J Immunol. 2013;77:413-18.
- [CrossRef] [PubMed] [Google Scholar]






