Translate this page into:
Cytokine gene polymorphisms in type I and type II reactions in Hansen's disease
Correspondence Address:
Nikhil Moorchung
Department of Pathology and Dermatology, Armed Forces Medical College, Pune, Maharashtra
India
| How to cite this article: Pragasam V, Vasudevan B, Moorchung N. Cytokine gene polymorphisms in type I and type II reactions in Hansen's disease. Indian J Dermatol Venereol Leprol 2020;86:482-488 |
Abstract
Introduction: Leprosy or Hansen's disease is a chronic debilitating disease caused by Mycobacterium leprae. Host genetics are believed to strongly influence the course of the disease. It is known that cytokines play an important role in leprosy and cytokine gene polymorphisms probably influence the course of the disease.
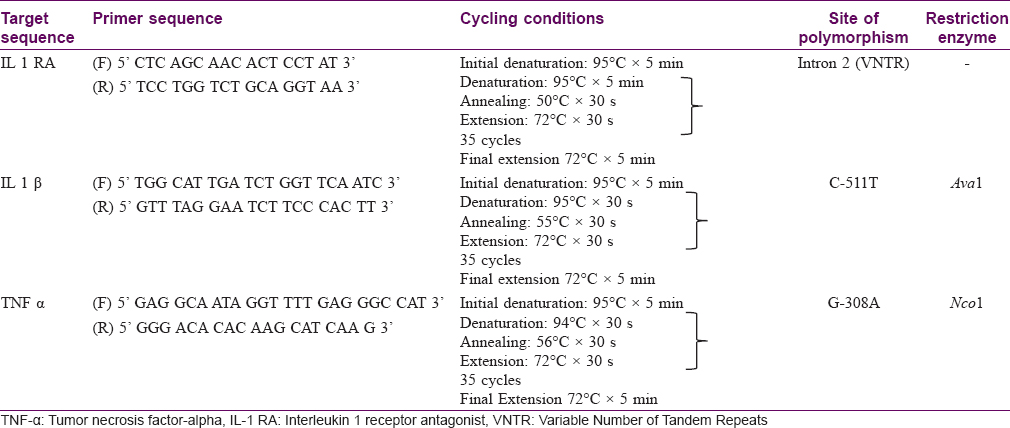
Methods: In the present study, we evaluated 70 patients with leprosy and 243 controls. DNA was extracted from the peripheral blood and genotyping was done for the following polymorphisms: IL-1 RA intron 2, IL-1β-511 C/T and TNF-α A/G.
Results: A strong association of TNF-α-308 G/A polymorphism with Hansen's disease with both genotypes and alleles was found. However, no correlation was identified between the other two polymorphisms and Hansen's disease. A strong association between the IL-1β gene polymorphisms and the type of reactions seen in leprosy was found. In contrast, the other two polymorphisms did not show any such association.
Limitations: Genetic polymorphisms are association studies. They are not a direct reflection of the transcriptome or proteome and this is a major limitation of this study.
Conclusion: In conclusion, cytokine gene polymorphisms appear to influence the susceptibility and course of Hansen's disease. An evaluation of the cytokine levels in the skin during lepra reactions would confirm this observation. Possibly, in future, this would be a guide to therapeutic decisions in cases of lepra reactions.
Introduction
Leprosy or Hansen's disease is a chronic debilitating disease caused by an obligate intracellular pathogen—Mycobacterium leprae (M. leprae). The clinical spectrum of the disease varies and it depends on the host immunity; one pole is tuberculoid leprosy in which the patients have a strong cellular immune response and the other pole is lepromatous leprosy (LL) where patients have a limited cellular immune response leading to a disseminated disease with diffuse infiltration of skin and nerves and bacilli-loaded foamy macrophages. Between the two poles, the majority of patients are borderline classified as borderline tuberculoid (BT), mid-borderline (BB) or borderline lepromatous (BL).[1],[2]
Factors influencing the type of leprosy are not well understood. Host genetics are believed to strongly influence the course of the disease. Cytokines play a critical role in triggering host-pathogen interaction in leprosy. The onset of tuberculoid leprosy is correlated with cytokine production by Th1 cells (IFN γ productions) and lepromatous leprosy is correlated with a Th 2 response (IL-4 production). Besides IFN and IL-4, other cytokines produced by T cells such as tumor necrosis factor-alpha (TNF-α), IL-6 and IL-10 and by macrophages such as IL-1 family also regulate M. leprae cell-mediated and humoral immune responses.[3],[4],[5],[6],[7]
Leprosy reactions are immune inflammatory-related phenotypes that may occur before diagnosis, during treatment or after Multi Drug Therapy (MD T). Lepra reactions require immediate treatment to prevent permanent disabilities. There are 2 major types of lepra reactions. Type I reaction is characterized by acute inflammation of preexisting skin lesions or by the appearance of new lesions and/or neuritis. Systemic symptoms are rare. Type I reactions are seen in patients who have an unstable immune response (BT, BB and BL forms)[8] or in patients with tuberculoid leprosy.[9],[10] Erythema nodosum leprosum is the main presentation of type II reaction which predominantly affects patients with leprosy showing Th2 immune response and high bacterial load. It is classified more toward the lepromatous pole (BB, BL, and LL) of the disease. Type II reaction is characterized by increased levels of TNF-α and immune complex–associated vasculitis, panniculitis and uveitis.
Cytokine gene polymorphisms have recently attracted considerable interest since it has been discovered that different alleles of cytokine genes are associated with different immunomodulatory diseases.[11],[12] Genes encoding cytokines and related molecules harbor polymorphic regions which are considered to alter gene transcription and thereby influence inflammatory processes in response to infectious disease.[13],[14] Some important cytokines that are known to harbor polymorphic regions are encoded by the genes of the interleukin-1 cluster and TNF-α.
The variability in clinical responses in patients with Hansen's disease is related to the number of cytokines secreted during the disease. Because type I and type II reactions are related to the Th 1 and Th 2 responses, it is logical to assume that cytokine secretion would influence the development of reactions. Because cytokine secretion would be influenced by genetic polymorphisms, it would be possible to predict the clinical response based on the genetic polymorphisms. Sousa et al. found an association between IL-6 gene polymorphisms and type II reactions.[15] We were unable to find any previous studies evaluating TNF-α and/or IL-1β gene polymorphisms and type I and type II reactions.
In this prospective case-control study, we attempted to evaluate the genetic polymorphisms of the TNF-α, IL-1 RA and the IL-1β genes in patients of Hansen's disease and compare the same with a control population with no evidence of Hansen's disease. We also attempted to evaluate the same genetic polymorphisms in patients showing reactions and compare the same with patients who did not show any reaction.
Methods
In the present study, we evaluated 70 patients with leprosy and 243 controls. The controls were age and sex matched and they were healthy individuals with no history of leprosy. The entire spectrum of Hansen's disease was included including tuberculoid, lepromatous, borderline tuberculoid, borderline lepromatous, borderline leprosy, pure neuritic and histoid leprosy. We also included patients with deformities which included claw hand, lagopthalmos and foot drop. Finally, cases who had undergone a reaction after therapy were also included in the study. These included patients with the type I and II reactions, neuritic and late reversal reactions. Ethical clearance was obtained for the study from the ethical committee of Command Hospital Southern Command.
Two milliliter of blood was collected from all these patients and controls. Genomic DNA was retrieved from the peripheral blood by using a standard kit (Quiagen).
The IL-1 RA intron 2 polymorphism was analyzed by a polymerase chain reaction. The polymerase chain reaction products were analyzed by electrophoresis on 2% agarose gel stained with ethidium bromide. The variable number of tandem repeats of an 86 bp fragment in the intron 2 was amplified. Five alleles were characterized based upon the number of repeats. Allele 1 (4 repeats) was 410 bp, allele 2 (2 repeats) was 240 bp, allele 3 (5 repeats) was 500 bp, allele 4 (3 repeats) was 325 bp and allele 5 (6 repeats) was 595 bp in length. Allele 2 was the proinflammatory allele.
For restriction fragment length polymorphism analysis of the 511 IL-1β polymorphism, the polymerase chain reaction products were first amplified. The confirmation of a successful polymerase chain reaction was done by running the products on a 2% agarose gel. If the products were present, they were digested with 10 U of Ava 1 at 37°C for 3 h. Fragments were analyzed by electrophoresis on 3% agarose gel and stained with ethidium bromide. This yielded products of 304 bp (TT allele), 190 bp and 104 bp (CC allele) and 304, 190 and 104 bp (CT allele). The T allele was the proinflammatory allele.
TNF-α gene polymorphisms were genotyped by a polymerase chain reaction and restriction fragment length polymorphism analysis. The polymerase chain reaction products were first amplified. The confirmation of a successful polymerase chain reaction was done by running the products on a 2% agarose gel. The products were digested with 10 U of Nco 1 at 37°C for 15 min. Fragments were analyzed by electrophoresis on 15% polyacrylamide gel and stained with ethidium bromide. This yielded products of 107 bp (AA allele), 87 bp and 20 bp (GG allele) and 107, 87 and 20 bp (AG allele). The A allele was considered proinflammatory.
The primer sequences are given in [Table - 1]. Polymerase chain reaction conditions are noted below. All polymerase chain reaction reactions were performed in an Eppendorf Thermal Cycler (MJ Research Inc, Waltham MA).

Statistical analysis
The sample size was calculated as 31 using the EPI INFO software. The results of the study were statistically analyzed using SPSS 16.0 statistical package program (Statistical Package for Social Sciences, Lead Technologies Inc, USA). The descriptive data were given as mean and standard deviation. The Chi-square test was used to compare the differences between groups. A value of P < 0.05 was considered to indicate statistical significance.
Results
Demographic profile
A total of 70 patients were evaluated in the study; their age ranged from 20 years to 65 years with an average of 32 years. There were 243 controls recruited in the study with no history of Hansen's disease in the past; their age ranged from 21 years to 45 years with a mean of 34 years. All 70 patients were men. Of the 243 controls, 240 were men and 3 were women. Informed consent was taken from all the patients.
Clinical diagnosis
The clinical diagnosis of the patients is shown in [Figure - 1]. In addition, all the patients were on multidrug therapy and no other therapeutic intervention was performed during the course of the study. The diagnosis of leprosy was confirmed by clinical examination, histopathology and slit-skin smear examination.
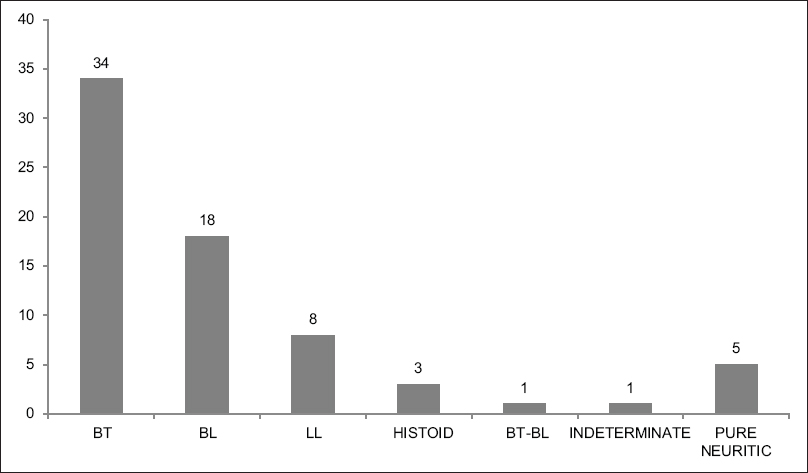 |
| Figure 1: Bar Chart showing the distribution of the patients with the different subtypes of Hansen's disease |
Reaction
The types of reactions seen in different patients are shown in [Figure - 2]. In addition, it must be mentioned that all the patients who had reactions were treated by standard protocols.
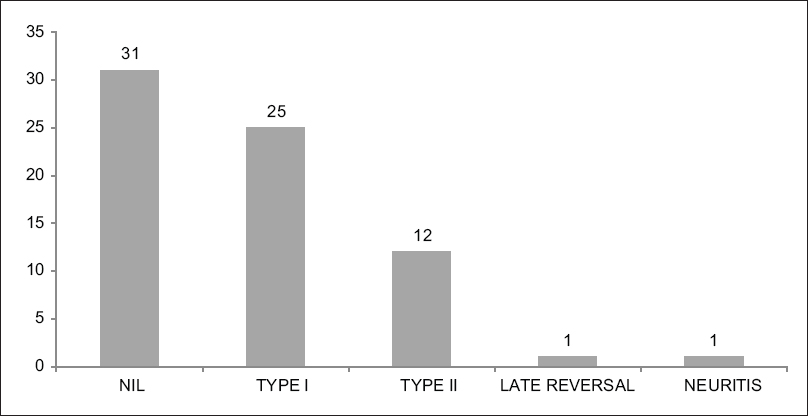 |
| Figure 2: Bar Chart showing the distribution of the different types of reactions in patients with Hansen's disease |
Deformities
The types of deformities seen in different patients are shown in [Figure - 3].
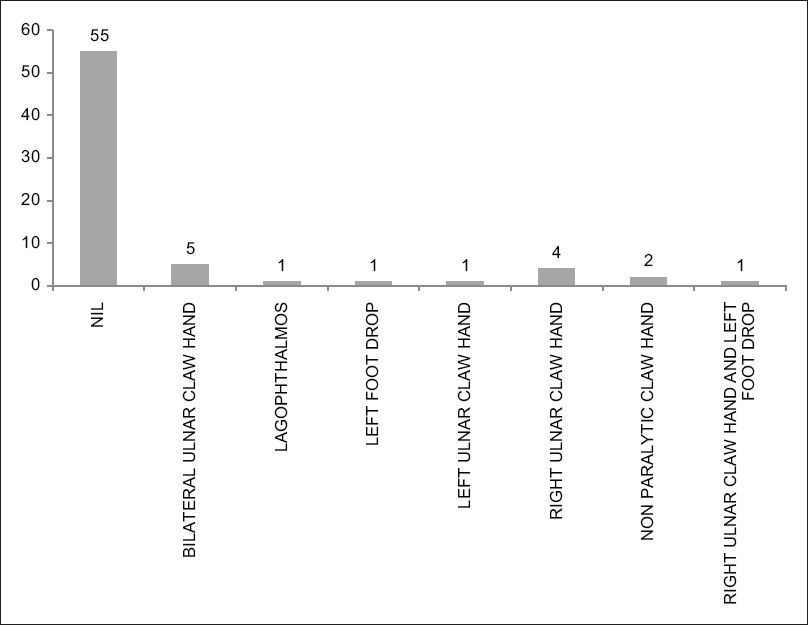 |
| Figure 3: Bar Chart showing the distribution of the different types of deformities in patients with Hansen's disease |
Tumor necrosis factor-alpha—308 A/G polymorphism—Association of genetic polymorphisms—Cases versus controls
The G allele was seen in 397 (81.6%) controls and 71 (50.7%) patients. In contrast, the proinflammatory A allele was seen in 89 (18.3%) controls and 69 (49.3%) patients. The GG genotype was seen in 163 (67.1%) controls and 4 (5.7%) patients. In contrast, the AA genotype was seen in only 9 (3.7%) controls and 3 (4.3%) patients. The AG genotype was seen in 71 (29.2%) controls and 63 (90.0%) patients [Table - 2].
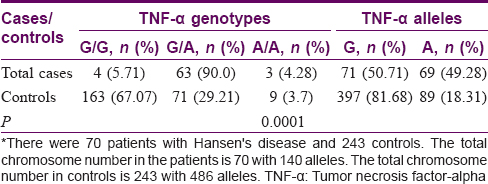
The A allele was more common in the cases of Hansen's disease as compared to the controls. The AG genotype was more common in the cases as opposed to the controls.
In controls, all the frequencies of tested genotypes and alleles were in Hardy–Weinberg equilibrium. There was a strong association of TNF-α-308 G/A polymorphism with Hansen's disease with both genotypes and allele [Table - 2] as the frequencies of genotypes were significantly different between patients and controls.
Interleukin-1 receptor antagonist polymorphism—Association of genetic polymorphisms—Cases versus controls
The A allele was seen in 368 (75.7%) controls and 100 (71.4%) patients. In contrast, the proinflammatory B allele was seen in 118 (24.3%) controls and 40 (28.6%) patients. The AA genotype was seen in 149 (61.3%) controls and 38 (54.9%) patients. In contrast, the BB genotype was seen in 24 (6.1%) controls and 8 (11.4%) patients. The AB genotype was seen in 70 (28.8%) controls and 24 (34.9%) patients [Table - 3].
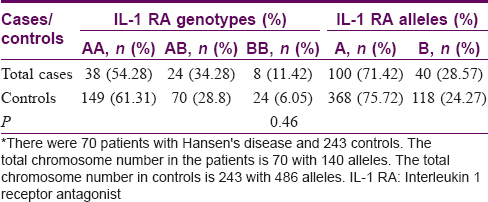
In controls, all the frequencies of tested genotypes and alleles were in Hardy–Weinberg equilibrium. There was no association between the different IL-1 RA alleles with the cases and the controls (P = 0.46).
Interleukin-1β polymorphism— Association of genetic polymorphisms—Cases versus controls
The C allele was seen in 184 (37.9%) controls and 47 (33.6%) patients. In contrast, the proinflammatory T allele was seen in 302 (62.1%) controls and 93 (66.4%) patients. The CC genotype was seen in 26 (10.7%) controls and 9 (12.9%) patients. The CT genotype was seen in 132 (54.3%) controls and 29 (41.4%) patients. Finally, the TT genotype was seen in 85 (35.0%) controls and 32 (45.7%) patients [Table - 4]. There was no correlation between the IL-1β polymorphisms and cases of Hansen's disease (P = 0.413).
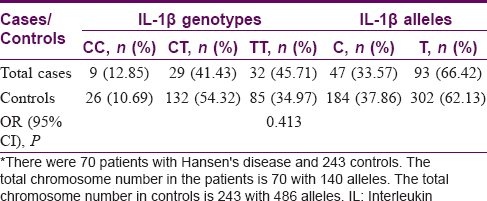
In controls, all the frequencies of tested genotypes and alleles were in Hardy–Weinberg equilibrium. There was no association of IL-1β-511 C/T polymorphism with Hansen's disease with both genotypes and alleles [Table - 3].
Association of genetic polymorphisms with the presence of a reaction
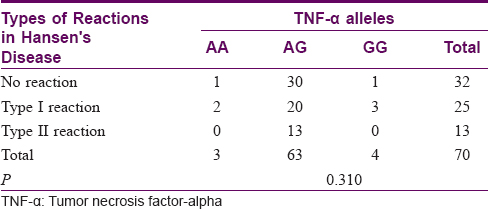
Association of tumor necrosis factor-alpha genetic polymorphisms with type I and type II reactions
There was no association between the TNF-α gene polymorphisms and the type of reaction (P = 0.310). The results are tabulated in [Table - 5].
Association of interleukin-1 receptor antagonist genetic polymorphisms with type I and type II reactions
There was no association between the IL-1 RA gene polymorphisms and the type of reaction (P = 0.830). The results are tabulated in [Table - 6].
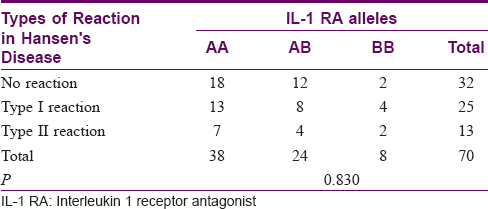
Association of interleukin-1β genetic polymorphisms with type I and type II reactions
There was a strong association between the IL-1β gene polymorphisms and the type of reaction (P = 0.009). The results are tabulated in [Table - 7].

Discussion
It has been reported in various studies[16],[17],[18],[19],[20] that the TNF-308A allele has a protective effect against the development of the disease. Similar findings have been reported in Nepal.[21] However, studies conducted in the Indian and Thai populations show a strong association of the A allele with the development of the disease.[22],[23] In the present study, we found a strong association between the A allele of the TNF-308 polymorphism and the susceptibility to leprosy. Our study suggests that the association of the A allele varies between populations and, in the Indian population, there is a strong association with the susceptibility to the development of leprosy.
We did not find an association between the IL-1 RA alleles and the susceptibility to leprosy. In a study involving 47 patients with leprosy, Settin et al. found a significant association of the A allele with the susceptibility to leprosy.[24] The association of IL-1 RA polymorphisms with inflammatory diseases is well known.[25],[26] However, in the Indian population, the relation between IL-1 RA and inflammatory diseases is less marked.[27],[28] In addition, we did not find an association between the IL-1β-511 C/T genetic polymorphisms and the susceptibility to leprosy. Studies performed in the Indian population have shown a lack of association between the IL-1β-511 C/T genetic polymorphisms and susceptibility to inflammatory diseases involving the skin.[29] There are several reasons for this. One hypothesis is that the IL-1β-511 C/T polymorphism is a nonfunctional polymorphism as suggested.[30],[31] However, Reich et al. have shown that the IL-1β-511 C/T is functional with the IL-1β-511 C/C genotype producing more IL-1 RA which is an antiinflammatory cytokine.[32] It has been shown that for some reason, the IL-1β present in the psoriatic skin though increased in amount in experimental models is functionally inactive.[33] This would probably explain the lack of an association between the IL-1 gene polymorphisms and leprosy.
There was a strong correlation between the IL-1β genetic polymorphisms and the lepra reactions. There was no association between the other two polymorphisms and the lepra reactions. The TT allele is proinflammatory and the CC allele is antiinflammatory. In the present study, it was seen that the TT allele predominated in the cases with both the type I and II lepra reactions. It is likely that an increased amount of IL-1β is secreted in cases who have lepra reactions. This would account for the exaggerated response which occurs in lepra reactions which is cytokine-mediated. However, it is surprising that the TNF-308 polymorphism was not associated with lepra reactions. TNF-α is believed to be the primary cytokine which regulates the rest of the cytokines in the inflammatory cascade. It is likely that the sample size was not adequate to evaluate this polymorphism.
We had a total of 15 patients who had deformities. However, because the number of cases was small, we could not perform a statistical analysis to evaluate whether the genetic polymorphisms were associated with the deformities.
We believe that this study provides an insight into the pathogenesis of lepra reactions. The polymorphism analysis suggests that there is an increase in the amount of cytokine produced. This finding has been confirmed in a study which measured the levels of the cytokines during reaction states and stated that the levels of TNF-α and IL-1β are elevated in leprosy reaction states.[34] These cytokines are responsible for the aggravation of the lepra reactions. Perhaps, cytokine inhibitors if administered would decrease the severity and duration of the reactions.
The limitation of this study is two. First, any study involving genetic polymorphisms is an association study. Transcriptomics and proteomics give a real-time picture of the changes during the course of the disease. However, in this study, evaluation of the cytokine levels would have been of little or no utility because the levels fluctuate considerably in the body and a single point measurement would not necessarily have given, a true picture. The second limitation was the sample size which appeared small. However, statistical software confirmed that the sample size was adequate considering the rarity of the disease.
Conclusion
In conclusion, an association of cytokine gene polymorphisms with Hansen's disease and the lepra reactions was found. An evaluation of the cytokine levels in the skin during the lepra reactions would confirm this observation. Possibly, in the future, this would be a guide to therapeutic decisions in lepra reactions.
Limitations of the study
Genetic polymorphisms are association studies. The DNA polymorphism may or may not have a relation to the transcriptome or the proteome in the tissue at that particular time. This would be a major limitation of this study.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form, the patients have given their consent for their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
| 1. |
Lockwood DN. Leprosy in the new millennium. J Med Microbiol 2000;49:301-3.
[Google Scholar]
|
| 2. |
Goulart IM, Penna GO, Cunha G. Immunopathology of leprosy: The complexity of the mechanisms of host immune response to Mycobacterium leprae. Rev Soc Bras Med Trop 2002;35:365-75.
[Google Scholar]
|
| 3. |
Fafutis-Morris M, Guillen-Vargas CM, Navarro-Fierro S, Morales-Ortiz R, Armendariz-Borunda J. Serum IL-1ra is elevated in lepromatous leprosy patients. Int J Lepr Other Mycobact Dis 1999;67:287-91.
[Google Scholar]
|
| 4. |
Abbas AK, Murphy KM, Sher A. Functional diversity of helper T lymphocytes. Nature 1996;383:787-93.
[Google Scholar]
|
| 5. |
Ahmed AA, Nordlind K, Schultzberg M, Lidén S. Interleukin-1 alpha- and beta-, interleukin-6- and tumour necrosis factor-alpha-like immunoreactivities in chronic granulomatous skin conditions. Acta Derm Venereol 1994;74:435-40.
[Google Scholar]
|
| 6. |
Lima MC, Pereira GM, Rumjanek FD, Gomes HM, Duppre N, Sampaio EP, et al. Immunological cytokine correlates of protective immunity and pathogenesis in leprosy. Scand J Immunol 2000;51:419-28.
[Google Scholar]
|
| 7. |
Bleharski JR, Li H, Meinken C, Graeber TG, Ochoa MT, Yamamura M, et al. Use of genetic profiling in leprosy to discriminate clinical forms of the disease. Science 2003;301:1527-30.
[Google Scholar]
|
| 8. |
Scollard DM, Adams LB, Gillis TP, Krahenbuhl JL, Truman RW, Williams DL. The continuing challenges of leprosy. Clin Microbiol Rev 2006;19:338-81.
[Google Scholar]
|
| 9. |
Kar HK, Sharma P. New lesions after MDT in PB and MB leprosy: A report of 28 cases. Indian J Lepr 2008;80:247-55.
[Google Scholar]
|
| 10. |
Stefani MM, Martelli CM, Gillis TP, Krahenbuhl JL; Brazilian Leprosy Study Group. In situ type 1 cytokine gene expression and mechanisms associated with early leprosy progression. J Infect Dis 2003;188:1024-31.
[Google Scholar]
|
| 11. |
Mansfield JC, Holden H, Tarlow JK, Di Giovine FS, McDowell TL, Wilson AG, et al. Novel genetic association between ulcerative colitis and the anti-inflammatory cytokine interleukin-1 receptor antagonist. Gastroenterology 1994;106:637-42.
[Google Scholar]
|
| 12. |
Clay FE, Cork MJ, Tarlow JK, Blakemore AI, Harrington CI, Lewis F, et al. Interleukin 1 receptor antagonist gene polymorphism association with lichen sclerosus. Hum Genet 1994;94:407-10.
[Google Scholar]
|
| 13. |
Barker JN, Mitra RS, Griffiths CE, Dixit VM, Nickoloff BJ. Keratinocytes as initiators of inflammation. Lancet 1991;337:211-4.
[Google Scholar]
|
| 14. |
Höhler T, Grossmann S, Stradmann-Bellinghausen B, Kaluza W, Reuss E, de Vlam K, et al. Differential association of polymorphisms in the TNF alpha region with psoriatic arthritis but not psoriasis. Ann Rheum Dis 2002;61:213-8.
[Google Scholar]
|
| 15. |
Sousa AL, Fava VM, Sampaio LH, Martelli CM, Costa MB, Mira MT, et al. Genetic and immunological evidence implicates interleukin 6 as a susceptibility gene for leprosy type 2 reaction. J Infect Dis 2012;205:1417-24.
[Google Scholar]
|
| 16. |
Santos AR, Almeida AS, Suffys PN, Moraes MO, Filho VF, Mattos HJ, et al. Tumor necrosis factor promoter polymorphism (TNF2) seems to protect against development of severe forms of leprosy in a pilot study in Brazilian patients. Int J Lepr Other Mycobact Dis 2000;68:325-7.
[Google Scholar]
|
| 17. |
Moraes MO, Duppre NC, Suffys PN, Santos AR, Almeida AS, Nery JA, et al. Tumor necrosis factor-alpha promoter polymorphism TNF2 is associated with a stronger delayed-type hypersensitivity reaction in the skin of borderline tuberculoid leprosy patients. Immunogenetics 2001;53:45-7.
[Google Scholar]
|
| 18. |
Santos AR, Suffys PN, Vanderborght PR, Moraes MO, Vieira LM, Cabello PH, et al. Role of tumor necrosis factor-alpha and interleukin-10 promoter gene polymorphisms in leprosy. J Infect Dis 2002;186:1687-91.
[Google Scholar]
|
| 19. |
Franceschi DS, Mazini PS, Rudnick CC, Sell AM, Tsuneto LT, Ribas ML, et al. Influence of TNF and IL10 gene polymorphisms in the immunopathogenesis of leprosy in the South of Brazil. Int J Infect Dis 2009;13:493-8.
[Google Scholar]
|
| 20. |
Cardoso CC, Pereira AC, Brito-de-Souza VN, Duraes SM, Ribeiro-Alves M, Nery JA, et al. TNF-308G>A single nucleotide polymorphism is associated with leprosy among Brazilians: A genetic epidemiology assessment, meta-analysis, and functional study. J Infect Dis 2011;204:1256-63.
[Google Scholar]
|
| 21. |
Sapkota BR, Macdonald M, Berrington WR, Misch EA, Ranjit C, Siddiqui MR, et al. Association of TNF, MBL, and VDR polymorphisms with leprosy phenotypes. Hum Immunol 2010;71:992-8.
[Google Scholar]
|
| 22. |
Roy S, McGuire W, Mascie-Taylor CG, Saha B, Hazra SK, Hill AV, et al. Tumor necrosis factor promoter polymorphism and susceptibility to lepromatous leprosy. J Infect Dis 1997;176:530-2.
[Google Scholar]
|
| 23. |
Vejbaesya S, Mahaisavariya P, Luangtrakool P, Sermduangprateep C. TNF alpha and NRAMP1 polymorphisms in leprosy. J Med Assoc Thai 2007;90:1188-92.
[Google Scholar]
|
| 24. |
Settin A, Nassar S, Abdel-Latif A, Elbaz R, El-Mongy S, Hassan A, et al. Association of cytokine gene polymorphism with susceptibility and clinical types of leprosy. Int J Health Sci (Qassim) 2007;1:25-33.
[Google Scholar]
|
| 25. |
Carter MJ, di Giovine FS, Jones S, Mee J, Camp NJ, Lobo AJ, et al. Association of the interleukin 1 receptor antagonist gene with ulcerative colitis in Northern European Caucasians. Gut 2001;48:461-7.
[Google Scholar]
|
| 26. |
Donaldson P, Agarwal K, Craggs A, Craig W, James O, Jones D, et al. HLA and interleukin 1 gene polymorphisms in primary biliary cirrhosis: Associations with disease progression and disease susceptibility. Gut 2001;48:397-402.
[Google Scholar]
|
| 27. |
Moorchung N, Srivastava AN, Gupta NK, Ghoshal UC, Achyut BR, Mittal B, et al. Cytokine gene polymorphisms and the pathology of chronic gastritis. Singapore Med J 2007;48:447-54.
[Google Scholar]
|
| 28. |
Achyut BR, Moorchung N, Srivastava AN, Gupta NK, Mittal B. Risk of lymphoid follicle development in patients with chronic antral gastritis: Role of endoscopic features, histopathological parameters, CagA status and interleukin-1 gene polymorphisms. Inflamm Res 2008;57:51-6.
[Google Scholar]
|
| 29. |
Moorchung N, Vasudevan B, Chatterjee M, Mani NS, Grewal RS. Interleukin-1 gene polymorphisms and their relation with NFκB expression and histopathological features in psoriasis. Indian J Dermatol 2015;60:432-8.
[Google Scholar]
|
| 30. |
Bidwell J, Keen L, Gallagher G, Kimberly R, Huizinga T, McDermott MF, et al. Cytokine gene polymorphism in human disease: On-line databases. Genes Immun 1999;1:3-19.
[Google Scholar]
|
| 31. |
Pociot F, Mølvig J, Wogensen L, Worsaae H, Nerup J. A taqI polymorphism in the human interleukin-1 beta (IL-1 beta) gene correlates with IL-1 beta secretion in vitro. Eur J Clin Invest 1992;22:396-402.
[Google Scholar]
|
| 32. |
Reich K, Mössner R, König IR, Westphal G, Ziegler A, Neumann C, et al. Promoter polymorphisms of the genes encoding tumor necrosis factor-alpha and interleukin-1beta are associated with different subtypes of psoriasis characterized by early and late disease onset. J Invest Dermatol 2002;118:155-63.
[Google Scholar]
|
| 33. |
Cooper KD, Hammerberg C, Baadsgaard O, Elder JT, Chan LS, Taylor RS, et al. Interleukin-1 in human skin: Dysregulation in psoriasis. J Invest Dermatol 1990;95:24S-26S.
[Google Scholar]
|
| 34. |
Sarno EN, Grau GE, Vieira LM, Nery JA. Serum levels of tumour necrosis factor-alpha and interleukin-1 beta during leprosy reactional states. Clin Exp Immunol 1991;84:103-8.
[Google Scholar]
|
Fulltext Views
5,141
PDF downloads
2,631





