Translate this page into:
Posterior reversible encephalopathy syndrome and intracerebral hemorrhage in an adult patient with Henoch-Schönlein purpura
2 Department of Intensive Care Unit, 306 Hospital of PLA, Beijing, China
Correspondence Address:
Shichao Lu
Department of Dermatology, 306 Hospital of PLA, 9 Anxiang North Rd, Chaoyang District, Beijing 100101
China
| How to cite this article: Liu J, Zong L, Gao J, Lu S. Posterior reversible encephalopathy syndrome and intracerebral hemorrhage in an adult patient with Henoch-Schönlein purpura. Indian J Dermatol Venereol Leprol 2019;85:502-505 |
Sir,
Henoch-Schönlein purpura (or IgA vasculitis) is a systemic small-vessel vasculitis that usually affects the skin, joints, gastrointestinal tract, and kidneys. The involvement of the central nervous system is uncommon in this condition. Herein, we present a rare case of Henoch-Schönlein purpura with posterior reversible encephalopathy syndrome and intracerebral hemorrhage.
An 18-year-old man was admitted to the 306 Hospital of PLA (Beijing, China) with a 1-week history of palpable purpura on the limbs [Figure - 1], arthralgia, and abdominal pain. He had no medical history of hypertension or epilepsy. Laboratory examination showed the following: white blood cell count of 14.8 × 10[9]/L (80% neutrophils), platelet count 359.0 × 10[9]/L, C-reactive protein 73.7 mg/L (normal, 0–8 mg/L), erythrocyte sedimentation rate 16 mm/h (normal, 0–15 mm/h), antistreptolysin O 379 IU/mL (normal, 0–200 IU/mL), and D-dimer 2608 ng/mL (normal, <255 ng/mL). Serum IgG, IgA, and IgM levels were within normal limits, and complements 3 and 4, and liver and kidney function tests were normal. Antinuclear antibody and antineutrophil cytoplasmic antibodies were negative. Urine analysis results indicated no significant abnormalities. Occult blood in stools was positive. A skin biopsy revealed leukocytoclastic vasculitis [Figure - 2]a with IgA deposits [Figure - 2]b, and thus Henoch-Schönlein purpura was diagnosed. Then prednisolone (0.5 mg/kg) and clindamycin were administered. However, his abdominal pain did not alleviate and the rash progressed from the limbs to the trunk and auriculae. The dose of prednisolone was then increased to 1 mg/kg.
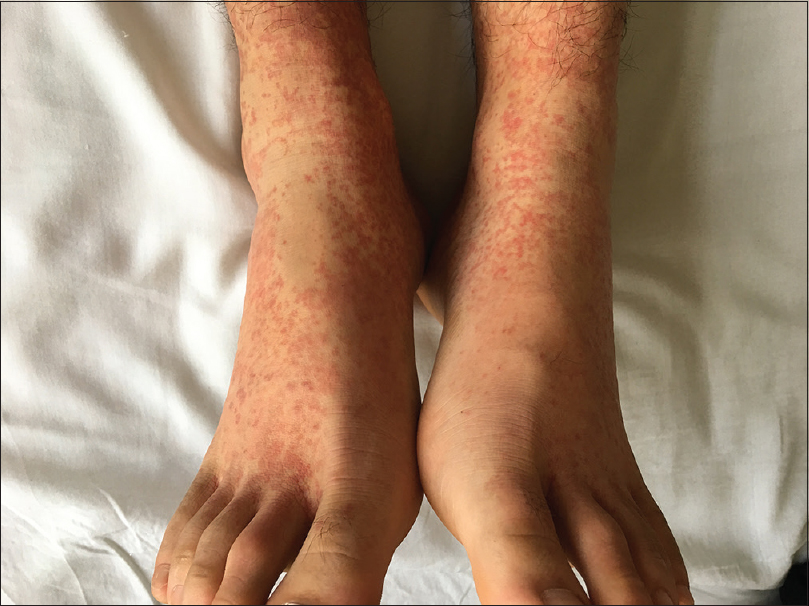 |
| Figure 1: Palpable purpura present on the patient's lower limbs on admission |
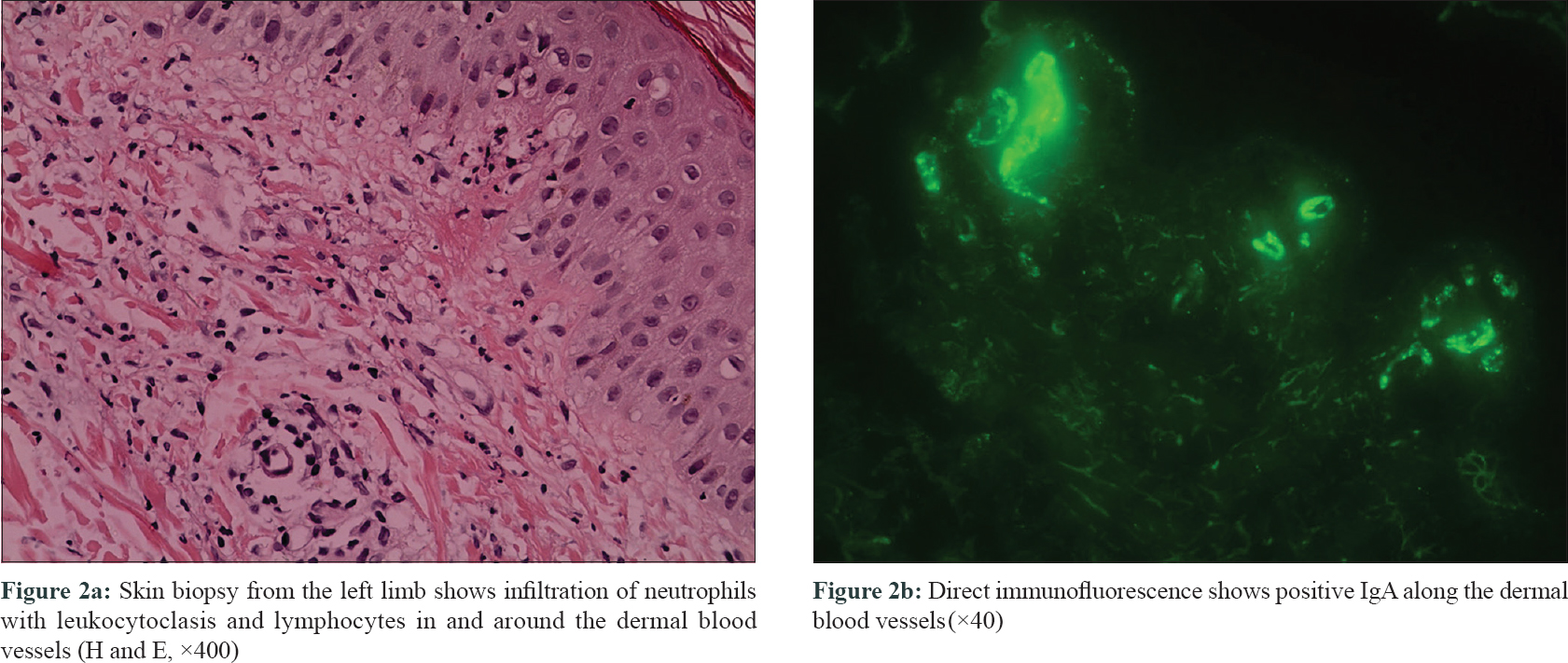 |
| Figure 2 |
On post-admission day 10, he presented with sudden headache and visual disturbances, followed by generalized tonic–clonic seizures lasting for a minute, which resolved spontaneously with postictal sleepiness. Blood pressure and heart rate were 160/100 mmHg and 100 beats/min, respectively. An emergency computed tomography of the brain did not reveal hemorrhage, cerebral edema, or signs of herniation. Brain magnetic resonance imaging performed 12 h after onset showed acute right frontal hemorrhage [Figure - 3]a and signal abnormalities in the cortico-subcortical areas of bilateral occipital lobes [Figure - 3]b, [Figure - 3]c, [Figure - 3]d, but magnetic resonance angiogram showed no arteriovenous malformation. Repeat D-dimer level was elevated to 9173 ng/mL. These findings were consistent with the diagnosis of posterior reversible encephalopathy syndrome and intracerebral hemorrhage. Urapidil hydrochloride, mannitol, and prednisolone (1.5 mg/kg) were rapidly administered. However, repeat computed tomography of brain revealed an increase in hematoma size and the patient presented with progressive left limb weakness and mental status deterioration. Minimally invasive puncture and drainage were performed immediately.
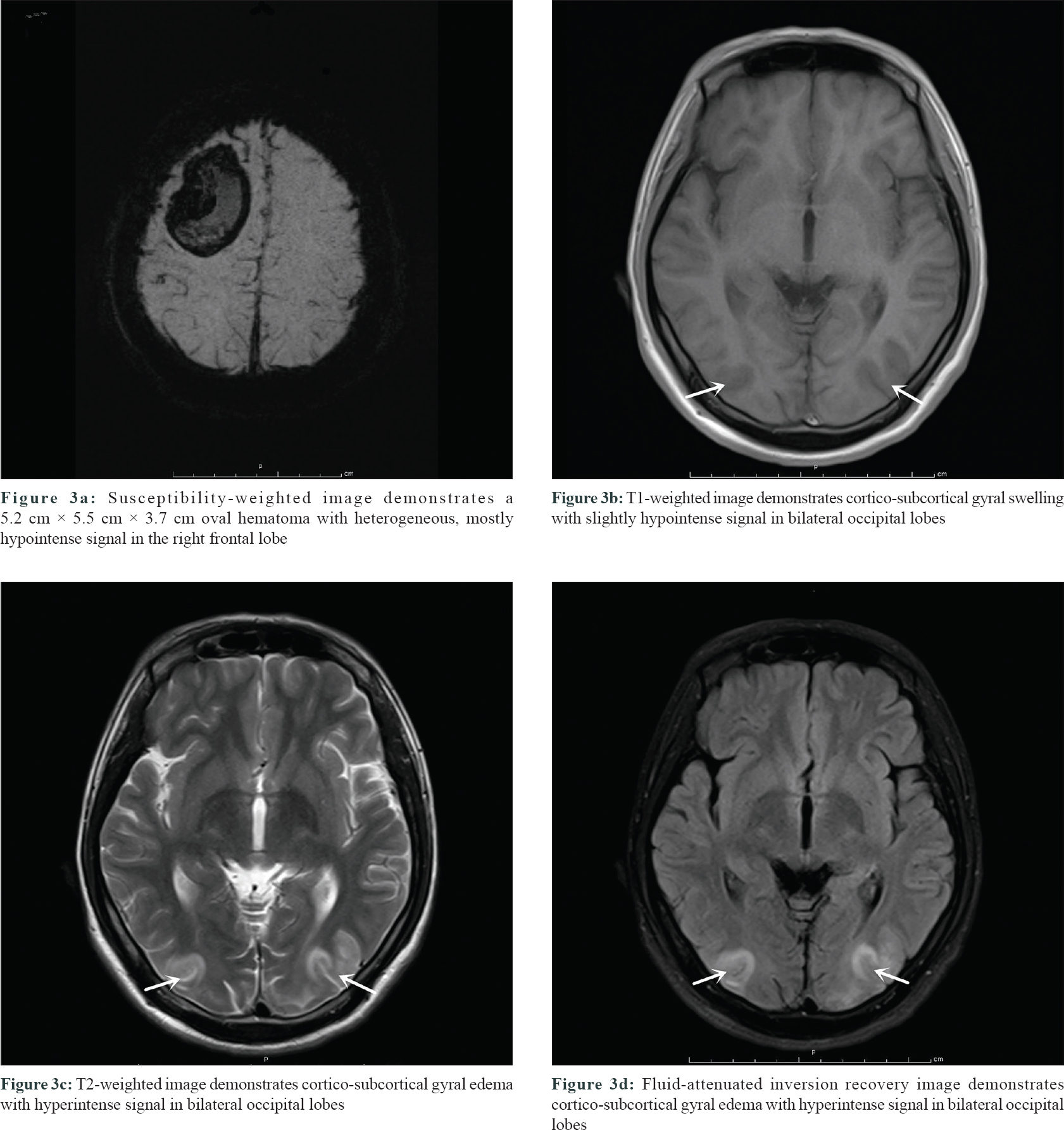 |
| Figure 3 |
Following surgery, D-dimer level was within the range of 1794–2010 ng/mL, and head computed tomography scan showed a decrease in hematoma size. At postoperative day 10, a blood test demonstrated the normalization of D-dimer, high blood pressure and abdominal pain resolved, and the prednisolone dose was gradually tapered. One month later, the patient had only mild weakness of the left limb at discharge. At 1-year follow-up, the patient was not on any medication and had no neurologic dysfunction. A repeat brain magnetic resonance imaging showed a 6.0 × 3.3 × 4.2-cm 3 lesion in the right frontal lobe (T1-weighted image showing a hypointense signal, T2-weighted image showing a hyperintense signal, and diffusion-weighted image showing a hypointense signal).
Central neurological involvement in Henoch-Schönlein purpura is rare (0.7%–8% of cases).[1] Its manifestations include cerebrovascular disease (edema, ischemia, infarction, and hemorrhage), hypertensive encephalopathy and posterior reversible encephalopathy syndrome, seizures, optic neuropathies, headache, and behavioral changes.[2] In a study that involved totally 112 patients with Henoch-Schönlein purpura, only 1 case developed posterior reversible encephalopathy syndrome.[1] Of patients with central neurological manifestations, 21% exhibit sequelae.[2] Magnetic resonance imaging is superior to computed tomography in detecting early lesions in posterior reversible encephalopathy syndrome and cerebral hemorrhage.[2] Clinicians must be aware of these neurologic complications and avoid delayed diagnosis and treatment.
Posterior reversible encephalopathy syndrome is a clinical and radiographic syndrome that clinically presents with headaches, altered consciousness, visual disturbances, and seizures and most commonly affects the parieto-occipital region.[3] In an article published in August 2018, the authors reported that from 1990 to 2017, there were totally 23 well-documented cases of IgA vasculitis with posterior reversible encephalopathy syndrome in the literature.[4] We performed a detailed review of the literature and identified no studies investigating the cutaneous pointers of posterior reversible encephalopathy syndrome. Although the pathophysiology is complex, in our case, posterior reversible encephalopathy syndrome and cerebral hemorrhage were probably due to endothelial dysfunction caused by systemic vasculitis and breakdown of the blood–brain barrier resulting from sudden hypertension.[2] We also cannot rule out a possible contributing role of corticosteroid therapy in increasing blood pressure and thereby potentially triggering posterior reversible encephalopathy syndrome through its effect on mineralocorticoid receptors.[5] Therefore, aggressive control of blood pressure and inflammatory response in patients with a severe disease course can help prevent the occurrence of neurological complications. In case of deterioration in patients with cerebral hemorrhage, neurosurgical intervention should be considered. The prognosis of Henoch-Schönlein purpura with posterior reversible encephalopathy syndrome is usually favorable and most patients had no neurological sequelae.[6] The reported sequelae included visual impairment, verbal disabilities, focal neurologic dysfunction, and epilepsy.[2]
Previous studies have demonstrated that injured endothelial cells in Henoch-Schönlein purpura expressed tissue factor and stimulated the coagulation.[7],[8] As a marker of hyperfibrinolysis, the D-dimer levels were significantly higher during the acute phase than during the convalescent phase and the levels significantly correlated with the total clinical score of Henoch-Schönlein purpura.[9],[10] In our case, a continuous increase in D-dimer level during the acute phase was also noted. This finding suggested that D-dimer can be used as an indicator of disease activity and can enable clinicians to evaluate treatment effectiveness. Nevertheless, additional studies are required to confirm this inference.
In conclusion, neurologic complications often occur in patients with severe Henoch-Schönlein purpura, and blood pressure and D-dimer level should be closely monitored. The early recognition and treatment of these complications is critical in achieving a good prognosis in patients with this condition.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form, the patient has given his consent for his images and other clinical information to be reported in the journal. The patient understand that name and initials will not be published and due efforts will be made to conceal identity, but anonymity cannot be guaranteed.
Acknowledgement
We would like to express our gratitude to Zhifeng Yang for the invaluable help in carrying out the neurosurgical intervention.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
| 1. |
Pacheva IH, Ivanov IS, Stefanova K, Chepisheva E, Chochkova L, Grozeva D, et al. Central nervous system involvement in Henoch-Schonlein purpura in children and adolescents. Case Rep Pediatr 2017;2017:5483543.
[Google Scholar]
|
| 2. |
Bérubé MD, Blais N, Lanthier S. Neurologic manifestations of Henoch-Schönlein purpura. Handb Clin Neurol 2014;120:1101-11.
[Google Scholar]
|
| 3. |
Hinchey J, Chaves C, Appignani B, Breen J, Pao L, Wang A, et al. A reversible posterior leukoencephalopathy syndrome. N Engl J Med 1996;334:494-500.
[Google Scholar]
|
| 4. |
Milani GP, Bianchetti MG, Lava SA. Posterior reversible encephalopathy syndrome in immunoglobulin A-associated vasculitis. Neurol Neurochir Pol 2018;52:634-5.
[Google Scholar]
|
| 5. |
Zhou J, Zheng H, Zhong X, Wu D, Wang M, Tang X, et al. Reversible posterior encephalopathy syndrome in children with nephrotic syndrome. Nephrology (Carlton) 2015;20:849-54.
[Google Scholar]
|
| 6. |
Roth C, Ferbert A. Posterior reversible encephalopathy syndrome: Long-term follow-up. J Neurol Neurosurg Psychiatry 2010;81:773-7.
[Google Scholar]
|
| 7. |
Besbas N, Erbay A, Saatçi U, Ozdemir S, Bakkaloglu A, Ozen S, et al. Thrombomodulin, tissue plasminogen activator and plasminogen activator inhibitor-1 in Henoch-Schönlein purpura. Clin Exp Rheumatol 1998;16:95-8.
[Google Scholar]
|
| 8. |
Brendel-Müller K, Hahn A, Schneppenheim R, Santer R. Laboratory signs of activated coagulation are common in Henoch-Schönlein purpura. Pediatr Nephrol 2001;16:1084-8.
[Google Scholar]
|
| 9. |
Yilmaz D, Kavakli K, Ozkayin N. The elevated markers of hypercoagulability in children with Henoch-Schönlein purpura. Pediatr Hematol Oncol 2005;22:41-8.
[Google Scholar]
|
| 10. |
Hong J, Yang HR. Laboratory markers indicating gastrointestinal involvement of Henoch-Schönlein purpura in children. Pediatr Gastroenterol Hepatol Nutr 2015;18:39-47.
[Google Scholar]
|
Fulltext Views
3,001
PDF downloads
1,556





