Translate this page into:
Anticoagulants in dermatology
Correspondence Address:
Arun C Inamadar
Department of Dermatology, Venereology and Leprosy, Shri B. M. Patil Medical College, Hospital and Research Center, BLDE University, Vijayapur - 586 103, Karnataka
India
| How to cite this article: Adya KA, Inamadar AC, Palit A. Anticoagulants in dermatology. Indian J Dermatol Venereol Leprol 2016;82:626-640 |
Abstract
Anticoagulants are the cornerstone of treatment of venous thromboembolism associated with various medical conditions and surgical procedures. They act on different steps of the coagulation pathway and are broadly categorized into heparins, vitamin K antagonists, and inhibitors of thrombin and factor Xa. The classification is evolving as newer and better oral and parenteral anticoagulants are being added. Anticoagulants in dermatology are important not only for their therapeutic application in cutaneous thrombotic dermatoses such as livedoid vasculitis, purpura fulminans, superficial and deep venous thrombosis and others but also for their use in non-thrombotic dermatoses such as lichen planus, recurrent oral aphthosis, chronic urticaria and several others. Further, the use of anticoagulants for any indication is associated with various adverse effects with dermatologic manifestations including specific reactions such as warfarin-induced skin necrosis, heparin-induced thrombocytopenia and anticoagulant-associated cholesterol embolization syndrome.Introduction
Anticoagulants are a class of oral and parenteral drugs that inhibit the development and progression of clots by acting on different phases of the coagulation pathway [Figure - 1]. These drugs neither lyse clots nor influence the fibrinolytic pathway. Anticoagulants can be broadly classified into heparins, vitamin K antagonists (coumarins) and inhibitors of thrombin and factor Xa [Table - 1]. The first two classes of drugs have been the cornerstone of treatment in venous thromboembolism with the latter two reserved for patients with complications and those requiring intervention.[1]
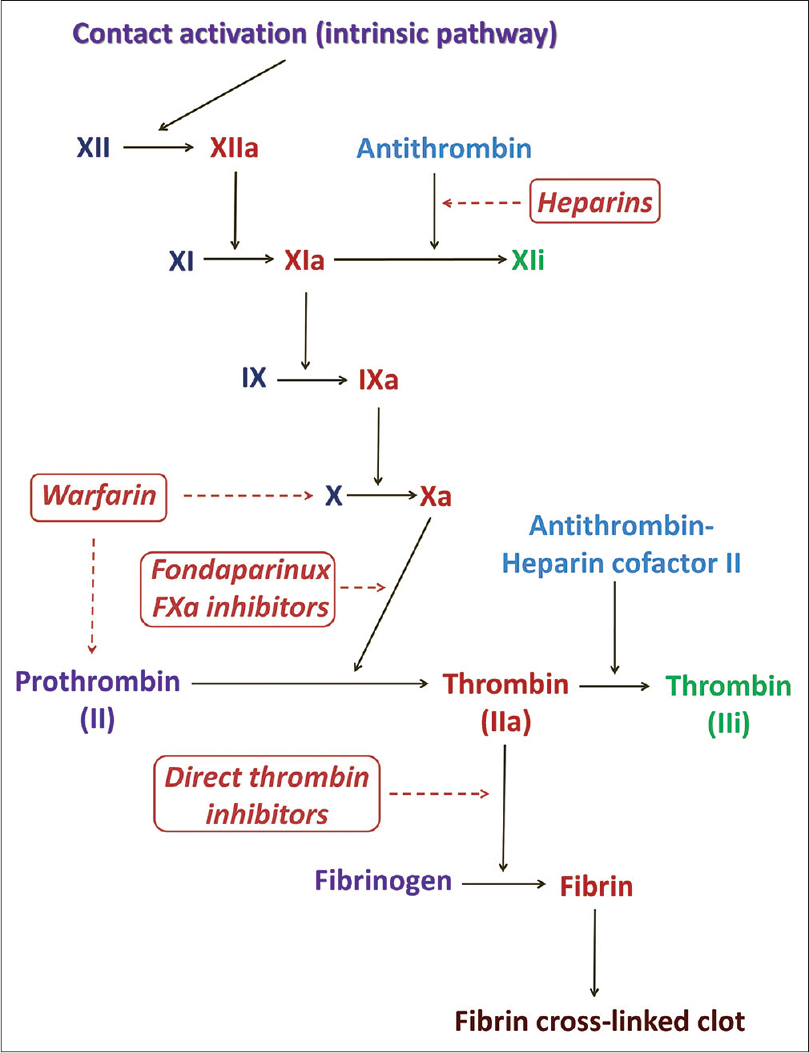 |
| Figure 1: The intrinsic pathway of the coagulation cascade and drugs acting at different steps therein. F: Factor, i: Inactive form, a: Active form |

Heparins [Table - 2] are rapidly acting parenteral anticoagulants and unfractionated heparin has been used for many years. In the last couple of decades, unfractionated heparin has largely been replaced by low molecular weight heparins as they are effective and safer and there is no need to routinely monitor their efficacy. Specific adverse effects associated with unfractionated heparin (see below) are rarely encountered with low molecular weight heparins. Heparins are widely used for the prevention and initial short-term treatment of venous thromboembolism.
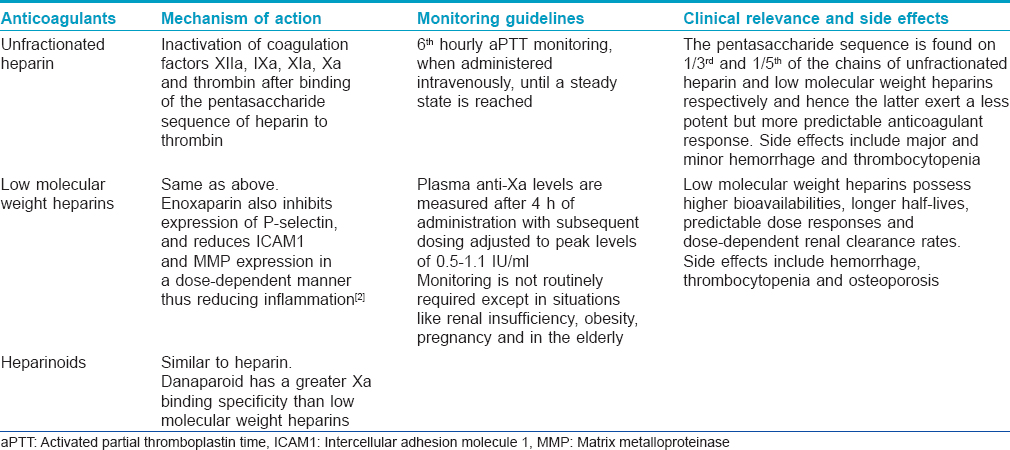
Vitamin K antagonists [Table - 3] are slow-acting and hence have no role when immediate anticoagulation is desired. They have a narrow therapeutic range and their efficacy needs frequent monitoring with determination of the international normalized ratio (INR). Vitamin K antagonists are currently used for the treatment and prophylaxis of venous thromboembolism in medical and surgical patients. Both heparins and vitamin K antagonists are used for the long-term prevention of stroke and systemic thromboembolism in patients with atrial fibrillation, for the secondary prevention and treatment of patients with acute coronary syndromes and in clinical situations requiring inhibition of intravascular clotting.[2],[3] Direct thrombin inhibitors and factor Xa inhibitors [Table - 3] are newer classes of anticoagulants with better characteristics and side effect profiles compared to the heparins and coumarins.
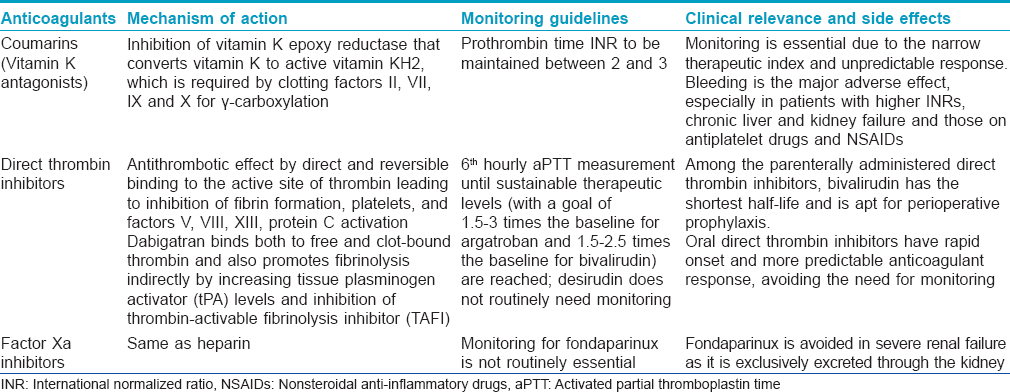
Dermatologically, anticoagulants are important both in terms of their therapeutic applications in various dermatoses as well as specific dermatological adverse effects associated with their usage [Table - 4]. Among the heparins, the low molecular weight heparins have been used in a variety of dermatological conditions and are not just limited to cutaneous thrombotic vasculopathies.
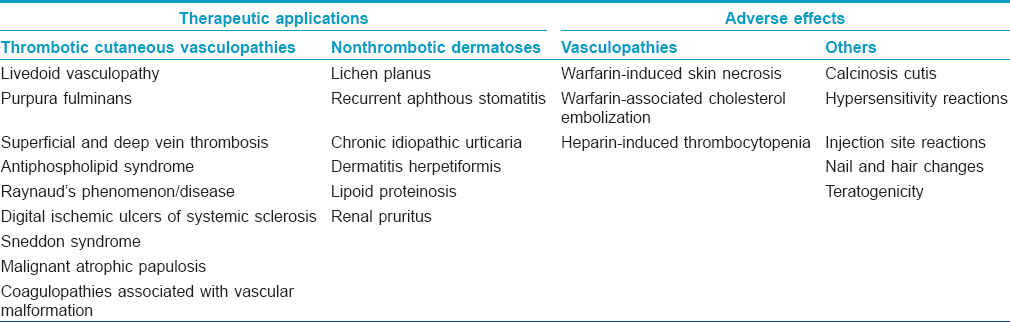
Therapeutic Applications of Anticoagulants in Dermatological Disorders
Thrombotic cutaneous vasculopathies
Livedoid vasculopathy
Livedoid vasculopathy or livedoid vasculitis is characterized by painful purpuric lesions involving the lower extremities that evolve into shallow ulcers which heal with stellate ivory-white scars [Figure - 2]. It is attributed to hypercoagulable states (idiopathic or associated with collagen vascular diseases, vascular thrombotic disorders and malignancies) resulting in microvascular thrombosis with consequent cutaneous ischemia and necrosis.[4] Various therapeutic modalities are employed in its treatment including antiplatelet drugs, fibrinolytics, vasodilators and intravenous immunoglobulins, with varied results.
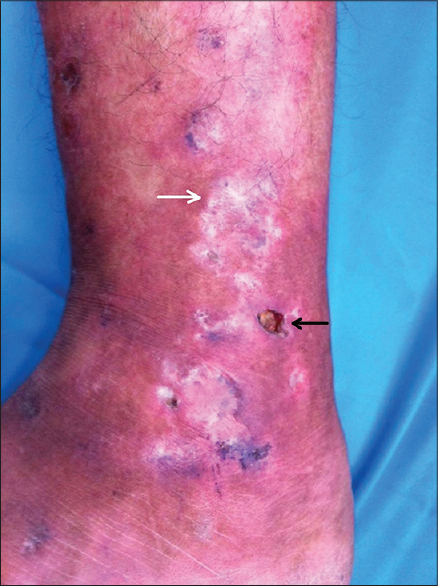 |
| Figure 2: Livedoid vasculopathy. Note the erythema, edema, ulcer (black arrow) and the characteristic ivory-white stellate scars (white arrow) |
Anticoagulant drugs, coumarins (warfarin) and heparins (unfractionated heparin and low molecular weight heparins) have proven beneficial both when used as monotherapy and in combination with other modalities listed above. Several anecdotal reports have upheld their role, especially in recalcitrant livedoid vasculopathy and livedoid vasculopathy associated with coagulation disorders, antiphospholipid syndrome and the like.[5],[6],[7],[8],[9],[10],[11] When warfarin is used, it is recommended to be started at lower doses maintaining the international normalized ratio between 1.5 and 2, and it may have to be continued for a few weeks after clinical resolution. However, the slow onset of action, need for frequent monitoring, narrow therapeutic index, frequent interactions with other drugs and difficulty in maintaining the desired international normalized ratio due to the patient's diet which may be rich in vitamin K have precluded the use of coumarins as first choice anticoagulants.[3]
Although both unfractionated heparin and low molecular weight heparins are used, the latter are currently preferred for livedoid vasculopathy because of their advantages although there still are concerns related to cost, need for parenteral administration and the risk of osteoporosis. The inherent anti-inflammatory action of low molecular weight heparins provides an added benefit in the treatment of livedoid vasculopathy [Figure - 3]. Hairston et al. reported the successful use of enoxaparin in two cases of livedoid vasculopathy that were not responding to anti-platelet and vasodilator drugs and advocated its use as a viable alternative in treating refractory livedoid vasculopathy.[12] Francès and Barete followed 16 patients of livedoid vasculopathy who were treated with various modalities, but only the response to anticoagulants was satisfactory to curative in the majority. They opined that anti-platelet drugs alone rarely induce complete remission and vitamin K antagonists and/or low molecular weight heparins are more frequently effective, the choice among them guided by cost concerns, risk-benefit ratio, quality-of-life parameters and associated co-morbidities.[13]
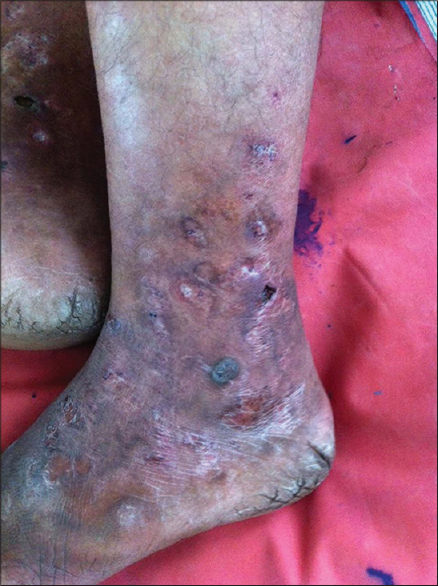 |
| Figure 3: Same patient [Figure 2] after 1 week of treatment with subcutaneous enoxaparin 75 mg twice daily. Note the reduction in erythema and edema, and the beginning of resolution |
There are no fixed guidelines regarding the dosage of anticoagulants in the treatment of livedoid vasculopathy. Hairston et al. used enoxaparin in a dose of 1 mg/kg twice daily (the dose used to treat active thrombosis) for 6 months followed by 1 mg/kg/day in one patient and 30 mg/day twice daily (peri-operative thrombo-prophylactic dose) in the other for 7 months. Di Giacomo et al. used enoxaparin 40 mg daily, dalteparin 5000 IU/day and unfractionated heparin 5000 IU 12th hourly in 5, 3 and 3 patients respectively.[5] Yang et al. treated 27 patients of atrophie blanche using unfractionated heparin. Nineteen (70%) of these patients who were administered a dose of 5000 IU daily had significant pain relief.[14] Hesse and Kutzner employed sub-thrombo-prophylactic doses of dalteparin (2500 IU/day for 14 days and then on alternate days until the ulcers healed) in 16 patients, nadroparin (2850 IU, 0.3 ml) in four patients and enoxaparin in two patients for an average duration of 7 weeks. Nineteen (86%) of these 22 patients showed complete remission.[7]
Among the newer non-vitamin K oral anticoagulants, rivaroxaban is currently undergoing a phase II multicentre trial in the treatment of livedoid vasculopathy.[15]
Purpura fulminans
The term purpura fulminans describes a heterogeneous group of acute, potentially fatal disorders characterized by extensive cutaneous necrosis and peripheral gangrene resulting from widespread thrombosis of the dermal capillaries and venules with hemorrhagic infarction of the surrounding tissues, associated with laboratory evidence of consumptive coagulopathy.[16] It occurs as a result of a relative deficiency of the protein C-mediated hemostasis mechanisms. Purpura fulminans is categorized into (a) neonatal purpura fulminans which is due to homozygous or compound heterozygous protein C or protein S deficiency that manifests shortly after birth, (b) acute infectious purpura fulminans that is due to an acquired protein C or protein S deficiency as a consequence of coagulative consumption of protein C, protein S and antithrombin III associated with sepsis-induced disseminated intravascular coagulation, and (c) post-infectious or idiopathic purpura fulminans due to the development of antibodies against protein S with consequent hypoactivation of the protein C pathway and dermal vascular hypercoagulability.[17]
The management of purpura fulminans includes an immediate phase therapy which aims at replenishing the pro- and anti-coagulant factors depleted in the setting of disseminated intravascular coagulation and a maintenance phase therapy aimed to prevent ongoing thrombosis. Immediate administration of fresh frozen plasma or protein C concentrate is the cornerstone of early phase management, along with supportive measures depending on the type and cause of purpura fulminans. Although anticoagulants help in reversing or preventing the progression of thrombosis, their use in the early phase is debated as there is an increased bleeding risk from reduced procoagulant factors due to disseminated intravascular coagulation. However, when purpura fulminans is accompanied by large venous thrombosis or central venous catheter thrombosis, heparin is usually necessary. It must be given along with fresh frozen plasma to reduce the risk of bleeding and avoid heparin resistance due to acquired antithrombin deficiency associated with severe sepsis.[18] Both unfractionated heparin and low molecular weight heparin can be used. Unfractionated heparin is recommended at a dose of 28 U/kg/h with a target anti-Xa level of 0.3–0.7 U/ml. The recommended dose of low molecular weight heparins is 1.0–1.5 mg/kg/dose every 12 h with a therapeutic target anti-Xa level of 0.5–1 U/ml.[19] Although it was not statistically significant, Kuppermann et al. observed that the mean number of digits and extremities with necrosis was less in those patients who received heparin within 72 h as against those who did not. In patients with only diffuse purpura at admission, a favorable outcome was much more frequent with heparin administration than without it.[20] Relapse can, however, occur if heparin is reduced or discontinued within several days of the initial response.[17],[21] In general, the post-infectious (idiopathic) form of purpura fulminans responds better to heparin with no tendency for recurrence while purpura fulminans associated with deficiency or inhibition of protein C responds better to protein C concentrate and is associated with recurrence.[21] Plasma exchange is also employed in idiopathic purpura fulminans to clear the autoantibodies. Thomson et al. successfully managed a case of post-varicella purpura fulminans with anticoagulation and plasma support and opined that timely anticoagulation may preempt the need for plasma exchange in such cases.[22] Macheret et al. noted a dramatic improvement in eschars after 1 week of initiation of dalteparin in their 6-month-old patient who presented with purpura fulminans with normal protein C and S levels. They advocate an empirical trial of anticoagulation to prevent the catastrophic complications of purpura fulminans and developmental delays in infants with this condition.[17]
Anticoagulants have a definite role in the long-term management of purpura fulminans, especially in the setting of deficiency or inactivation of protein C or S. Warfarin is recommended widely in this regard. If protein C is not concurrently administered as prophylaxis, the international normalized ratio needs to be maintained between 2.5 and 3.5. With protein C replacement, a smaller dose of warfarin (maintaining the international normalized ratio between 1.5 and 2.5) is recommended.[23] Warfarin therapy should be used with extreme caution since it can cause further depletion of protein C and S and may precipitate further microvascular thrombosis and purpura fulminans. Warfarin administration must therefore overlap with, and only be started after several days of anticoagulation with heparin to avoid warfarin-induced skin necrosis (see below) and other thrombotic complications.[19] Martinelli et al. in their 6-year-old patient with purpura fulminans with severe protein S deficiency on long-term warfarin prophylaxis noted recurrent warfarin-induced skin necrosis as the depletion of protein C (attributed to a relatively lower international normalized ratio target) outweighed its anticoagulant action. They switched over to rivaroxaban resulting in disappearance of skin necrosis by 1 year. Hence, they suggest rivaroxaban as an appropriate anticoagulant in patients with severe inherited protein S deficiency and warfarin-induced skin necrosis.[24] Long-term successful anticoagulation in purpura fulminans has also been achieved using low molecular weight heparins.[25]
Superficial venous thrombosis
Superficial venous thrombosis is a relatively under-reported clinical entity characterized by an acutely inflamed and tender cord-like thickening of superficial veins along with erythema, variable edema and tenderness of the surrounding skin. Superficial venous thrombosis commonly occurs in the lower limb, involving the greater saphenous vein, mostly in the setting of varicose veins, especially associated with old age, obesity and protein S deficiency. Superficial venous thrombosis may complicate or coexist with deep vein thrombosis or pulmonary embolism.[26]
Various treatment modalities for superficial venous thrombosis include the use of elastic stockings, non-steroidal anti-inflammatory drugs, anticoagulants and surgical treatment. Considering the risk of thromboembolic complications associated with superficial venous thrombosis and with superficial venous thrombosis being a cause for recurrent deep vein thrombosis, the use of anticoagulants (as monotherapy or as an adjuvant to surgical treatment) is frequently advocated as they act on thrombus formation and its propagation, the main pathophysiological events of the disease.[27]
Studies comparing heparins (unfractionated heparin and low molecular weight heparin) with anti-inflammatory agents have demonstrated significantly more favorable outcomes in terms of clinical resolution, recurrence and complications in those who received heparins.[28],[29],[30] Therapeutic doses, in general, have more advantages than prophylactic doses.[31] Ascer et al. administered full therapeutic doses of heparin in 20 patients with thrombophlebitis of the sapheno-femoral junction followed by warfarin as maintenance treatment. They observed prompt resolution, no episodes of pulmonary embolism, no recurrences and no complications of anticoagulation with a follow-up of 14 months.[32] However, Prandoni et al. did not note much difference between the fixed prophylactic dose and weight-adjusted therapeutic dose of nadroparin in terms of progression of superficial venous thrombosis and venous thrombo-embolic complications.[33] Topical formulations of heparin have also been studied but these were only preliminary studies whose conclusions need to be confirmed using appropriate study designs and adequate sample size.[34],[35]
A Cochrane review published in 2007 recommended non-steroidal anti-inflammatory drugs and low molecular weight heparin as the first option in the treatment of superficial venous thrombosis.[36] However, the second update on this review recommends prophylactic-dose fondaparinux given for 6 weeks as a valid therapeutic option for superficial venous thrombosis of the legs.[37] This review also included a large randomized, double-blind trial which found fondaparinux, administered subcutaneously at a dose of 2.5 mg once daily to be associated with significant reductions in symptomatic venous thromboembolism, superficial venous thrombosis extension and recurrence, with comparable rates of major bleeding relative to placebo.[38] The latest consensus guidelines from the American College of Chest Physicians also recommend prophylactic fondaparinux or low molecular weight heparin over no anticoagulation and fondaparinux over low molecular weight heparin for extensive superficial venous thrombosis of the legs.[39] Fondaparinux should be avoided in patients with renal disease, active bleeding, bacterial endocarditis and body weight <50 kg. Furthermore, prolonged administration warrants renal function monitoring and termination of therapy if creatinine clearance is <30 ml/min.[40] In patients with advanced renal insufficiency, unfractionated heparin is the drug of choice.[41]
Evidence for the use of coumarins in the treatment of superficial venous thrombosis is limited.[42]
Deep vein thrombosis
Deep vein thrombosis and pulmonary embolism are collectively included under venous thromboembolism. Deep vein thrombosis most commonly involves the deeper venous system of the lower extremities and is characterized clinically by erythema, edema, warmth, pain and tenderness. It needs prompt and aggressive treatment because of its potential for complications that include pulmonary embolism and recurrent deep vein thrombosis. Although previous episodes of deep vein thrombosis remain the single most important risk marker, old age, obesity, malignancy, hereditary thrombophilia and prolonged immobility associated with major surgery (especially orthopedic surgery) or hemiplegia are other important risk factors. In up to 50% of cases, however, no identifiable risk factors are seen and such “unprovoked” cases carry a high risk of recurrence.[43],[44]
The management of deep vein thrombosis involves prophylaxis in patients following major surgeries (such as total hip and knee replacement surgery), and treatment of the problem when it develops. Anticoagulants are the primary treatment modality in both these situations. Treatment involves anticoagulants in an initial acute phase ( first 5–10 days) followed subsequently by extended treatment. The conventional protocol involves the initial use of parenteral heparin (unfractionated heparin or low molecular weight heparin) or fondaparinux, transitioned to oral warfarin as long-term treatment. However, owing to the need for frequent monitoring with warfarin (and other disadvantages described above), non-vitamin K oral anticoagulants such as apixaban, edoxaban, rivaroxaban (factor Xa inhibitors) or dabigatran (direct thrombin inhibitors) have been studied and all have shown efficacies comparable to the conventional approach.[45]
Büller et al. found edoxaban to be non-inferior to warfarin both in terms of recurrent symptomatic venous thromboembolism and major or clinically relevant non-major bleeding.[46] A fixed-dose dabigatran regimen (150 mg twice daily for 6 months) was found comparable to warfarin in terms of efficacy and safety, without laboratory monitoring.[47],[48] Eighteen (50%) of the 36 patients in another study showed complete dissolution of the deep vein thrombosis over a period of 4.3 ± 4.3 months when dabigatran was used as monotherapy without preceding heparin.[49] Apixaban has also been administered as an extended anticoagulation therapy in patients with venous thromboembolism who had already completed 6–12 months of anticoagulation, in whom the treating physicians were uncertain about continuation of anticoagulant therapy. In comparison to placebo, apixaban reduced the risk of recurrent venous thromboembolism without increasing the rate of major bleeding.[50] Further, fixed-dose apixaban therapy has been found to be non-inferior to conventional therapy in acute venous thromboembolism and was associated with significantly less bleeding.[51]
For venous thromboembolism prophylaxis following major orthopedic surgeries (total hip replacement or total knee replacement), the American College of Chest Physicians recommends the use of low molecular weight heparin, fondaparinux, low-dose unfractionated heparin, adjusted-dose vitamin K antagonists, aspirin, or an intermittent pneumatic compression device for 10–14 days.[52] Based on the results of clinical trials, apixaban has been approved by the United State Food and Drug Administration for the primary prevention of deep vein thrombosis following hip and knee replacement surgeries.[53],[54],[55] Dabigatran has also been approved for this indication based on the results of a meta-analysis of data from various studies.[56] Although evidence is thus mounting in favour of non-vitamin K oral anticoagulants over conventional anticoagulants, certain clinical scenarios such as renal failure, malignancy and the presence of active bleeding at the time of presentation preclude their use as frontline medications in the management of deep vein thrombosis.[45]
As regards the duration of anticoagulation in venous thromboembolism, the “acute phase” is generally for 3 months. The duration of “extended anticoagulation” is primarily influenced by the long-term risk of recurrence and secondarily by the risk of bleeding and patient preference. Indefinite anticoagulation is practiced in patients with active cancer, a second unprovoked venous thromboembolism event and in any circumstance where the risk of recurrence after completion of active phase treatment remains unacceptably high.[57]
Antiphospholipid syndrome
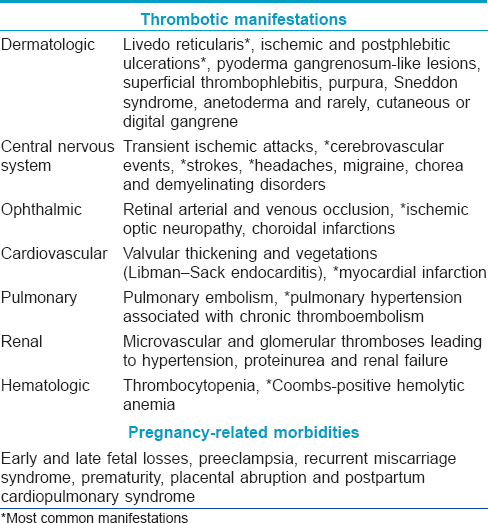
Antiphospholipid syndrome is an autoimmune acquired prothrombotic disorder characterized by recurrent arterial or venous thrombosis, pregnancy-related complications and circulating antiphospholipid antibodies. Persistent moderate-to-high positivity confirmed 12 weeks apart for at least one of anti-β2-glycoprotein 1 antibody, anticardiolipin antibody and lupus anticoagulant is necessary for a diagnosis of antiphospholipid syndrome. The secondary form of antiphospholipid syndrome is commonly associated with systemic lupus erythematosus. Cutaneous manifestations of antiphospholipid syndrome are myriad [Table - 5] and some of them correlate with involvement of the systemic vasculature (e.g., livedo reticularis is associated with cardiac and central nervous system thrombosis).[58] IgA anticardiolipin antibody positivity is an independent predictive factor for development of cutaneous lesions.[59] A catastrophic form of antiphospholipid syndrome with multifocal thromboses occurring simultaneously or in quick succession has also been described.[60]
Anticoagulants in antiphospholipid syndrome are mainly employed for the treatment of thrombotic episodes and secondary thromboprophylaxis, and debate continues as to whether all asymptomatic patients with antiphospholipid antibodies should be given prophylaxis. However, the risk of a thrombotic event in asymptomatic antiphospholipid syndrome is significantly high among those who are positive for lupus anticoagulant (especially the IgG isotype), those showing triple positivity and patients with an underlying autoimmune disorder and cardiovascular risk factors.[62] Although studies have shown mixed results, anti-platelet therapy with low-dose aspirin is commonly recommended in such cases; it may be combined with hydroxychloroquine in patients with concomitant systemic lupus erythematosus.[63],[64],[65] Anticoagulants as a primary thromboprophylactic measure are advocated, with administration of low molecular weight heparin to cover high-risk episodes such as infections, trauma, major surgeries and prolonged immobilization.[64] Use of low-dose oral anticoagulants has also been advocated.[66] No additional benefit was noted when low-dose aspirin was combined with warfarin as compared to low-dose aspirin alone. In fact, a greater number of bleeding episodes and lower patient compliance were noted in the combination treatment group.[67]
Warfarin is the mainstay of secondary thromboprophylaxis in the antiphospholipid syndrome. The intensity and duration of anticoagulation predominantly depend on whether the past thrombotic episode was venous or arterial. Although a higher intensity of anticoagulation may seem superior to moderate intensity, studies have not shown this.[68],[69],[70] For a past episode of arterial thrombosis, a more aggressive approach with higher intensity of anticoagulation (international normalized ratio >3) or combination antithrombotic therapy is recommended.[70],[71] Although some observations have shown the benefit of a limited duration of anticoagulation in a selected group of patients with antiphospholipid syndrome, as of now, indefinite secondary prophylaxis is recommended in both venous and arterial thrombosis as the re-thrombosis rate is significant, especially in the first 6 months of cessation of warfarin.[72],[73],[74]
Where there is resistance or intolerance to warfarin or where warfarin is contraindicated (e.g., pregnancy), heparin/low molecular weight heparins appear to be viable options.[62],[75],[76] In obstetric antiphospholipid syndrome, a combination of aspirin and heparin (or low molecular weight heparin) is recommended.[77] Aspirin should be started before conception or once a positive pregnancy test is obtained. Warfarin must be substituted with heparin or preferably a low molecular weight heparin, continued throughout pregnancy. Warfarin can be restarted postpartum once the therapeutic international normalized ratio has been achieved.[78]
Some small studies showed the potential usefulness of non-vitamin K oral anticoagulants in antiphospholipid syndrome and several large trials are ongoing.[79],[80],[81],[82],[83],[84] However, there is no consensus on the use of these agents as yet and warfarin remains the first-line secondary thromboprophylactic treatment in the antiphospholipid syndrome.
Other thrombotic cutaneous vasculopathies
Anticoagulants have also been used in Raynaud's phenomenon and digital ischemic ulcers of systemic sclerosis, Sneddon syndrome and malignant atrophic papulosis. Although calcium channel blockers and vasodilators are the first-line drugs in the management of Raynaud's phenomenon and digital ischemic ulcers of systemic sclerosis, enoxaparin has also been found to be useful in improving digital blood flow and progressive healing of digital ischemia.[85],[86] However, appropriate studies are required to confirm these findings before low molecular weight heparin can be used as an effective alternative. Sneddon syndrome (idiopathic, associated with primary antiphospholipid syndrome or associated with systemic lupus erythematosus with or without antiphospholipid antibodies) is characterized by generalized livedo reticularis with vascular thrombosis of the central nervous system commonly involving the middle and posterior cerebral arteries.[87] The current treatment for Sneddon syndrome is long-term anticoagulation with warfarin as in the antiphospholipid syndrome.[88] Malignant atrophic papulosis or Köhlmeier–Degos disease is a multi-system thrombotic vasculopathy of unknown origin involving the skin, gastrointestinal tract and central nervous system, with characteristic papular cutaneous lesions that develop a typical central porcelain-white atrophy surrounded by telangiectasia. There is no effective treatment for Köhlmeier–Degos disease and various modalities such as anti-platelet, fibrinolytic, immunosuppressive and biological agents (eculizumab) as well as anticoagulants have been tried with varying results.[89],[90] Other situations where anticoagulant treatment is employed include the treatment of inadvertent injection of sclerosant into arteries, and prophylactically in Klippel–Trenaunay syndrome and coagulopathies associated with vascular malformations.
Non-thrombotic dermatoses
Lichen planus
There are several reports of the successful use of low molecular weight heparin in lichen planus, especially in the recalcitrant forms, owing to their anti-proliferative and immunomodulatory actions when employed in low doses. Very low doses have been shown to inhibit delayed hypersensitivity reactions and tumor necrosis factor α production, both of which are key elements in the pathogenesis of lichen planus.[91] Low molecular weight heparin was also shown to inhibit production of heparanase by activated CD4+ T-cells which allows them to penetrate the subendothelial basal lamina.[92] At a dose of 3 mg/week administered subcutaneously, enoxaparin led to recovery in 15 (71.4%) of 21 patients with lichen planus.[93] In comparison with oral prednisone, enoxaparin (5 mg/week) showed almost similar efficacy with fewer relapses and less side-effects in patients with disseminated lichen planus.[94] Enoxaparin has also proved beneficial in severe lichen planus not responding to conventional modalities. Thirteen (86.6%) of 15 patients showed significant improvement at the end of 1 month and 12 (80%) showed good improvement at the end of one study which employed 3 mg/week of enoxaparin for 20 weeks in lichen planus not responding to conventional treatment.[95] A case of severe ulcerative cutaneous lichen planus with concomitant hepatitis C infection was successfully treated with low molecular weight heparin.[96] Topical heparinoid has also been found effective in the treatment of cutaneous lichen planus not responding to topical steroids or tacrolimus.[97]
Persistent generalized lichen nitidus has been reported to be successfully managed with enoxaparin sodium as well.[98]
Recurrent aphthous stomatitis
Based on the effectiveness of low molecular weight heparin in oral lichen planus, a pilot exploratory trial involving thirty patients with recurrent aphthous stomatitis treated with 3 mg/week of enoxaparin, administered subcutaneously was undertaken by Ghaffari et al. A significant decline in the number, size, recurrences, intensity and duration of painful lesions was noted after 8 weeks of treatment.[99] Femiano et al. observed that the efficacy of systemic sulodexide was comparable to that of systemic prednisone with no significant adverse effects in patients with recurrent aphthous stomatitis not responding to topical corticosteroids.[100]
Chronic idiopathic urticaria
Chronic idiopathic urticaria is associated with activation of the coagulation cascade and fibrinolysis due to the involvement of eosinophils and tissue factor pathways with the resultant generation of thrombin. Plasma D-dimer levels (markers of coagulation cascade and fibrinolytic pathway activation) are seen to be elevated in chronic idiopathic urticaria which correlate with disease severity as well as reduced responsiveness to antihistamines.[101],[102] This is probably the basis for the effectiveness of low molecular weight heparin and fibrinolytics such as tranexamic acid in some cases of chronic idiopathic urticaria not responding to conventional therapeutic protocols. In a pilot study in patients with chronic idiopathic urticaria with persistently elevated plasma D-dimer who were unresponsive to antihistamines, there was marked improvement in 5 (62.5%) of 8 patients who were given nadroparin (11400 IU/day) and tranexamic acid (1 g thrice daily) for 2 weeks.[102]
Others
Heparin has also been used in certain recalcitrant cases of dermatitis herpetiformis, for cutaneous lesions of lipoid proteinosis (intralesional heparin) and in renal pruritus.[103],[104],[105],[106]
Dermatological Adverse Effects of Anticoagulants
Vasculopathies
Warfarin-induced skin necrosis
Warfarin-induced skin necrosis is an uncommon, potentially life-threatening condition. It is more common in obese women in their sixth and seventh decades, especially in the setting of major surgery. Warfarin-induced skin necrosis occurs within 3–6 days of administration of high doses of warfarin (≥10 mg) without heparin/low molecular weight heparin bridging. As warfarin exerts its action by inhibiting vitamin K-dependent clotting factors, a transient prothrombotic milieu is created initially after the administration of warfarin due to inactivation of these factors. Protein C and factor VII are most vulnerable as they have the lowest half-life among all the vitamin K dependent factors.[90],[107] This situation is amplified in the presence of predisposing factors such as inherent deficiency of protein C and/or other vitamin K-dependent clotting factors and infections, or the presence of a thrombophilic disorder.[107],[108]
The initial manifestation is sudden onset pain followed by the development of well-demarcated erythema that evolves to hemorrhagic bullae, necrosis and eschar formation. Areas with abundant fat such as the breasts, abdomen, hips and buttocks are predominantly involved. Histopathologically, fibrin thrombi are seen within the dermal and subcuticular vessels, with no or minimal inflammation.[90]
Treatment is aimed at reversing the effects of warfarin by the administration of vitamin K or protein C concentrates. Warfarin must be stopped and replaced by heparin/low molecular weight heparin. Warfarin may then be cautiously re-introduced, overlapping with heparin/low molecular weight heparin with the latter stopped once the desired international normalized ratio is achieved. Excessive necrosis and eschar formation may require surgical debridement and skin grafting.[108] Late-onset warfarin-induced skin necrosis in certain cases has been attributed to poor compliance with warfarin therapy, with its stoppage and resumption without heparin/low molecular weight heparin, acquired hypercoagulable states and abnormal liver function.[109],[110]
Anticoagulant-associated cholesterol embolization syndrome
Apart from arterial catheterization and thrombolytic therapy, the cholesterol embolization syndrome may also occur 4–8 weeks after anticoagulation therapy in patients with an underlying asymptomatic or severe atheromatous disease. Anticoagulation produces gradual dissolution of the clot overlying and stabilizing an atheromatous plaque. As a result, cholesterol crystals from the exposed plaque core can traverse through the circulation and lodge in small arterioles where they incite an inflammatory reaction leading to intravascular thrombosis, endothelial proliferation and fibrosis.
Clinical manifestations depend on the source of the emboli and the corresponding site of their lodgment. Frequent sources are the abdominal aorta, and iliac and femoral arteries. As a result, manifestations are commonly seen in the lower part of the body. The predominant cutaneous findings include livedo reticularis (most frequent), acrocyanosis (blue/purple toe syndrome) or acral gangrene with preserved peripheral pulses. Other lesions include nodules, infiltrated plaques and purpura. Systemic manifestations include sudden-onset hypertension, acute renal failure and gastrointestinal hemorrhage.
Management of cholesterol embolization is essentially individualized as there are no standard guidelines. Discontinuation of anticoagulants, administration of iloprost (parenteral) and antiplatelet drugs have been recommended. In the presence of laboratory evidence of inflammation (elevated C-reactive protein and erythrocyte sedimentation rate), systemic steroids are advised. Surgical modalities include endarterectomy and bypass or stent grafting. Lumbar sympathectomy is believed to relieve ischemic symptoms in the blue toe syndrome. Ischemia and necrosis may on occasion be severe enough to mandate amputation.[111],[112],[113]
Heparin-induced thrombocytopenia
Heparin-induced thrombocytopenia is an acquired, potentially dangerous hypercoagulability condition associated with the development of antibodies against heparin/platelet factor 4 complex which bind to platelet surface receptors resulting in their activation and aggregation and leading to thrombosis of veins, arteries or cutaneous microvasculature. Heparin-induced thrombocytopenia is slightly more common in women and post-surgical patients are especially predisposed.[90],[113] Although different classes of anti-heparin/platelet factor 4 antibodies are produced, IgG antibodies are important in producing thrombosis. However, only about 5–30% of cases with antibodies develop heparin-induced thrombocytopenia.[114]
The syndrome commonly begins 5–15 days after heparin administration and is more common with unfractionated heparin (especially bovine heparin) than with low molecular weight heparin; it may rarely be associated with fondaparinux.[115] Thrombotic complications develop in 20–50% of patients with venous thrombosis (pulmonary embolism, thrombosis of deep veins of the lower limbs or adrenal veins) being more common than arterial thrombosis (acute ischemia of the extremities, myocardial or cerebral infarctions).[114] Cutaneous microvascular involvement presents as a well-demarcated, tender, purpuric rash with a characteristic retiform configuration and minimal erythema/inflammation. Lesions can occur at the site of subcutaneous injection or at distant sites when heparin is infused intravenously, and may progress to cutaneous necrosis and eschar formation. Histopathologically, the involved vessels appear plugged with platelets without fibrin (“white clots”) or inflammation. Diagnosis of heparin-induced thrombocytopenia is based on clinical considerations, the platelet count and detection of anti-heparin/platelet factor 4 antibodies using an enzyme immunoassay. Significant vascular thrombosis may or may not be associated with thrombocytopenia and interpretation of platelet counts in established heparin-induced thrombocytopenia may at times be tricky if the pre-heparinization platelet count is unknown. A 50% reduction from the pre-treatment value is a better indicator of heparin-induced thrombocytopenia than the absolute platelet count. Polyclonal enzyme immunoassays lack specificity but are useful in excluding heparin-induced thrombocytopenia as they have a negative predictive value of 100%. More specific tests would be to estimate anti-heparin/platelet factor 4-IgG antibodies and confirmation with serotonin release or heparin-induced platelet activation assays.[90],[113],[114]
Treatment of heparin-induced thrombocytopenia involves cessation of heparin and anticoagulation with one of the direct thrombin inhibitors (lepirudin or argatroban), heparinoids (danaparoid) or fondaparinux.[116],[117],[118] The average duration of treatment is 4 and 12 weeks in asymptomatic patients and in those with thrombosis, respectively. Intravenous gammaglobulins and plasmapheresis can be alternatives in patients not responding to conventional measures. Data regarding the use of non-vitamin K oral anticoagulants in heparin-induced thrombocytopenia are lacking. Platelet transfusion is not recommended (unless the count is very low and associated with bleeding, or the patient is undergoing an intervention that carries risk of bleeding) as the transfused platelets may get activated, further complicating thrombosis.[114]
Other dermatological adverse effects of anticoagulants
Various cutaneous reactions may be observed at the injection site of heparins such as purpura, ecchymoses, necrosis, and infiltrated plaques.[119],[120] Calcinosis cutis has been reported to develop when calcium-containing low molecular weight heparin preparations are used.[121],[122] Heparins and other anticoagulants are also associated with hypersensitivity reactions such as urticaria, angioedema and Baboon syndrome, as also delayed hypersensitivity with patch test positivity.[119],[123] Telogen effluvium and nail changes (reduced growth, transverse bands and subungal hematomas) have been described in patients on heparin.[119],[124],[125] Heparins have been implicated in group 8 aplasia cutis congenita.[126]
Conclusion
It is important for dermatologists to have an in-depth knowledge of existing and emerging anticoagulants and their applications in various dermatological disorders. We may also come across patients on anticoagulant medications, where knowledge of these agents might help in formulating an appropriate line of management for their skin disorders without affecting their anticoagulation treatment. Obtaining information regarding what anticoagulant the patient is on and why, is especially prudent in dermatosurgery.[127],[128] Finally, being mindful of the specific cutaneous adverse effects related to their use is indispensable for their early identification and management.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
| 1. |
Alquwaizani M, Buckley L, Adams C, Fanikos J. Anticoagulants: A review of the pharmacology, dosing, and complications. Curr Emerg Hosp Med Rep 2013;1:83-97.
[Google Scholar]
|
| 2. |
Criado PR, Rivitti EA, Sotto MN, Valente NY, Aoki V, Carvalho JF, et al. Livedoid vasculopathy: An intringuing cutaneous disease. An Bras Dermatol 2011;86:961-77.
[Google Scholar]
|
| 3. |
Tsiara S, Pappas K, Boutsis D, Laffan M. New oral anticoagulants: Should they replace heparins and warfarin? Hellenic J Cardiol 2011;52:52-67.
[Google Scholar]
|
| 4. |
Adya KA, Inamadar AC, Palit A. Reticulate dermatoses. Indian J Dermatol 2014;59:3-14.
[Google Scholar]
|
| 5. |
Di Giacomo TB, Hussein TP, Souza DG, Criado PR. Frequency of thrombophilia determinant factors in patients with livedoid vasculopathy and treatment with anticoagulant drugs – A prospective study. J Eur Acad Dermatol Venereol 2010;24:1340-6.
[Google Scholar]
|
| 6. |
Deng A, Gocke CD, Hess J, Heyman M, Paltiel M, Gaspari A. Livedoid vasculopathy associated with plasminogen activator inhibitor-1 promoter homozygosity (4G/4G) treated successfully with tissue plasminogen activator. Arch Dermatol 2006;142:1466-9.
[Google Scholar]
|
| 7. |
Hesse G, Kutzner H. Therapeutic use of low molecular weight heparin for capillaritis alba. Phlebologie 2008;37:259-65.
[Google Scholar]
|
| 8. |
Cardoso R, Gonçalo M, Tellechea O, Maia R, Borges C, Silva JA, et al. Livedoid vasculopathy and hypercoagulability in a patient with primary Sjögren's syndrome. Int J Dermatol 2007;46:431-4.
[Google Scholar]
|
| 9. |
Osada S, Kimura Y, Kawana S. Case of livedoid vasculopathy with peripheral neuropathy successfully treated with low-dose warfarin. J Dermatol 2010;37:98-101.
[Google Scholar]
|
| 10. |
Davis MD, Wysokinski WE. Ulcerations caused by livedoid vasculopathy associated with a prothrombotic state: Response to warfarin. J Am Acad Dermatol 2008;58:512-5.
[Google Scholar]
|
| 11. |
Kavala M, Kocaturk E, Zindanci I, Turkoglu Z, Altintas S. A case of livedoid vasculopathy associated with factor V Leiden mutation: Successful treatment with oral warfarin. J Dermatolog Treat 2008;19:121-3.
[Google Scholar]
|
| 12. |
Hairston BR, Davis MD, Gibson LE, Drage LA. Treatment of livedoid vasculopathy with low-molecular-weight heparin: Report of 2 cases. Arch Dermatol 2003;139:987-90.
[Google Scholar]
|
| 13. |
Francès C, Barete S. Difficult management of livedoid vasculopathy. Arch Dermatol 2004;140:1011.
[Google Scholar]
|
| 14. |
Yang LJ, Chan HL, Chen SY, Kuan YZ, Chen MJ, Wang CN, et al. Atrophie blanche: A clinicopathological study of 27 patients. Chang Gung Med J 1991;14:237-45.
[Google Scholar]
|
| 15. |
Drabik A, Hillgruber C, Goerge T. A phase II multicenter trial with rivaroxaban in the treatment of livedoid vasculopathy assessing pain on a visual analog scale. JMIR Res Protoc 2014;3:e73.
[Google Scholar]
|
| 16. |
Levin M, Eley B, Faust SN. Purpura fulminans. In: Irvine A, Hoeger P, Yan A, editors. Harper's Textbook of Pediatric Dermatology. 3rd ed. Oxford: Wiley-Blackwell; 2011. p. 162.1-162.16.
[Google Scholar]
|
| 17. |
Macheret F, Pundi KN, Broomall EM, Davis DM, Rodriguez V, Brands CK. Empiric treatment of protracted idiopathic purpura fulminans in an infant: A case report and review of the literature. J Med Case Rep 2011;5:201.
[Google Scholar]
|
| 18. |
Chalmers E, Cooper P, Forman K, Grimley C, Khair K, Minford A, et al. Purpura fulminans: Recognition, diagnosis and management. Arch Dis Child 2011;96:1066-71.
[Google Scholar]
|
| 19. |
Price VE, Ledingham DL, Krümpel A, Chan AK. Diagnosis and management of neonatal purpura fulminans. Semin Fetal Neonatal Med 2011;16:318-22.
[Google Scholar]
|
| 20. |
Kuppermann N, Inkelis SH, Saladino R. The role of heparin in the prevention of extremity and digit necrosis in meningococcal purpura fulminans. Pediatr Infect Dis J 1994;13:867-73.
[Google Scholar]
|
| 21. |
Nolan J, Sinclair R. Review of management of purpura fulminans and two case reports. Br J Anaesth 2001;86:581-6.
[Google Scholar]
|
| 22. |
Thomson JJ, Retter A, Hunt BJ. Novel management of post varicella purpura fulminans owing to severe acquired protein S deficiency. Blood Coagul Fibrinolysis 2010;21:598-600.
[Google Scholar]
|
| 23. |
Goldenberg NA, Manco-Johnson MJ. Protein C deficiency. Haemophilia 2008;14:1214-21.
[Google Scholar]
|
| 24. |
Martinelli I, Bucciarelli P, Artoni A, Fossali EF, Passamonti SM, Tripodi A, et al. Anticoagulant treatment with rivaroxaban in severe protein S deficiency. Pediatrics 2013;132:e1435-9.
[Google Scholar]
|
| 25. |
Monagle P, Andrew M, Halton J, Marlar R, Jardine L, Vegh P, et al. Homozygous protein C deficiency: Description of a new mutation and successful treatment with low molecular weight heparin. Thromb Haemost 1998;79:756-61.
[Google Scholar]
|
| 26. |
Giannoukas A. Current management of superficial thrombophlebitis of the lower limb. Phlebolymphology 2013;20:127-32.
[Google Scholar]
|
| 27. |
SobreiraI ML, Yoshida WB, Lastória S. Superficial thrombophlebitis: Epidemiology, physiopathology, diagnosis and treatment. J Vasc Bras 2008;7:131-43.
[Google Scholar]
|
| 28. |
Titon JP, Auger D, Grange P, Hecquet JP, Remond A, Ulliac P, et al. Therapeutic management of superficial venous thrombosis with calcium nadroparin. Dosage testing and comparison with a non-steroidal anti-inflammatory agent. Ann Cardiol Angeiol (Paris) 1994;43:160-6.
[Google Scholar]
|
| 29. |
Superficial Thrombophlebitis Treated By Enoxaparin Study Group. A pilot randomized double-blind comparison of a low-molecular-weight heparin, a nonsteroidal anti-inflammatory agent, and placebo in the treatment of superficial vein thrombosis. Arch Intern Med 2003;163:1657-63.
[Google Scholar]
|
| 30. |
Rathbun SW, Aston CE, Whitsett TL. A randomized trial of dalteparin compared with ibuprofen for the treatment of superficial thrombophlebitis. J Thromb Haemost 2012;10:833-9.
[Google Scholar]
|
| 31. |
Marchiori A, Verlato F, Sabbion P, Camporese G, Rosso F, Mosena L, et al. High versus low doses of unfractionated heparin for the treatment of superficial thrombophlebitis of the leg. A prospective, controlled, randomized study. Haematologica 2002;87:523-7.
[Google Scholar]
|
| 32. |
Ascer E, Lorensen E, Pollina RM, Gennaro M. Preliminary results of a nonoperative approach to saphenofemoral junction thrombophlebitis. J Vasc Surg 1995;22:616-21.
[Google Scholar]
|
| 33. |
Prandoni P, Tormene D, Pesavento R; Vesalio Investigators Group. High vs. low doses of low-molecular-weight heparin for the treatment of superficial vein thrombosis of the legs: A double-blind, randomized trial. J Thromb Haemost 2005;3:1152-7.
[Google Scholar]
|
| 34. |
Górski G, Szopinski P, Michalak J, Marianowska A, Borkowski M, Geremek M, et al. Liposomal heparin spray: A new formula in adjunctive treatment of superficial venous thrombosis. Angiology 2005;56:9-17.
[Google Scholar]
|
| 35. |
Katzenschlager R, Ugurluoglu A, Minar E, Hirschl M. Liposomal heparin-spraygel in comparison with subcutaneous low molecular weight heparin in patients with superficial venous thrombosis. A randomized, controlled, open multicentre study. J Kardiol 2003;10:375-8.
[Google Scholar]
|
| 36. |
Di Nisio M, Wichers IM, Middeldorp S. Treatment for superficial thrombophlebitis of the leg. Cochrane Database Syst Rev 2007;2:CD004982.
[Google Scholar]
|
| 37. |
Di Nisio M, Wichers IM, Middeldorp S. Treatment for superficial thrombophlebitis of the leg. Cochrane Database Syst Rev 2013;4:CD004982.
[Google Scholar]
|
| 38. |
Decousus H, Prandoni P, Mismetti P, Bauersachs RM, Boda Z, Brenner B, et al. Fondaparinux for the treatment of superficial-vein thrombosis in the legs. N Engl J Med 2010;363:1222-32.
[Google Scholar]
|
| 39. |
Kearon C, Akl EA, Comerota AJ, Prandoni P, Bounameaux H, Goldhaber SZ, et al. Antithrombotic therapy for VTE disease: Antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012;141 2 Suppl: e419S-94S.
[Google Scholar]
|
| 40. |
Rosh AJ, Khait L, Rowe VL. Superficial Thrombophlebitis Treatment & Management. Available from: http://www.emedicine.medscape.com/article/463256-treatment#d11. [Last accessed on 2015 Jun 24].
[Google Scholar]
|
| 41. |
DeSancho MT, Pastores SM. Prophylactic dose fondaparinux for 6 weeks in superficial thrombophlebitis of the legs reduces the risk for symptomatic thromboembolic complications. Evid Based Med 2013;18:28-9.
[Google Scholar]
|
| 42. |
Belcaro G, Nicolaides AN, Errichi BM, Cesarone MR, De Sanctis MT, Incandela L, et al. Superficial thrombophlebitis of the legs: A randomized, controlled, follow-up study. Angiology 1999;50:523-9.
[Google Scholar]
|
| 43. |
Patel K, Chun LJ, Brenner BE. Deep Venous Thrombosis. Available from: http://www.emedicine.medscape.com/article/1911303-overview#a5. [Last accessed on 2015 Jun 28].
[Google Scholar]
|
| 44. |
Fanola CL. Current and emerging strategies in the management of venous thromboembolism: Benefit-risk assessment of dabigatran. Vasc Health Risk Manag 2015;11:271-82.
[Google Scholar]
|
| 45. |
Hillis C, Crowther MA. Acute phase treatment of VTE: Anticoagulation, including non-Vitamin K antagonist oral anticoagulants. Thromb Haemost 2015;113:1193-202.
[Google Scholar]
|
| 46. |
Hokusai-VTE Investigators, Büller HR, Décousus H, Grosso MA, Mercuri M, Middeldorp S, et al. Edoxaban versus warfarin for the treatment of symptomatic venous thromboembolism. N Engl J Med 2013;369:1406-15.
[Google Scholar]
|
| 47. |
Schulman S, Kearon C, Kakkar AK, Mismetti P, Schellong S, Eriksson H, et al. Dabigatran versus warfarin in the treatment of acute venous thromboembolism. N Engl J Med 2009;361:2342-52.
[Google Scholar]
|
| 48. |
Schulman S, Kakkar AK, Goldhaber SZ, Schellong S, Eriksson H, Mismetti P, et al. Treatment of acute venous thromboembolism with dabigatran or warfarin and pooled analysis. Circulation 2014;129:764-72.
[Google Scholar]
|
| 49. |
Fujino T, Yamazaki Y, Yamazaki A, Kabuki T, Kiuchi S, Kobayashi K, et al. Efficacy of dabigatran for dissolving deep vein thromboses in outpatients with a deteriorated general condition. Int Heart J 2015;56:395-9.
[Google Scholar]
|
| 50. |
Agnelli G, Buller HR, Cohen A, Curto M, Gallus AS, Johnson M, et al. Apixaban for extended treatment of venous thromboembolism. N Engl J Med 2013;368:699-708.
[Google Scholar]
|
| 51. |
Agnelli G, Buller HR, Cohen A, Curto M, Gallus AS, Johnson M, et al. Oral apixaban for the treatment of acute venous thromboembolism. N Engl J Med 2013;369:799-808.
[Google Scholar]
|
| 52. |
Guyatt GH, Akl EA, Crowther M, Gutterman DD, Schuünemann HJ; American College of Chest Physicians Antithrombotic Therapy and Prevention of Thrombosis Panel. Executive summary: Antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012;141 2 Suppl: 7S-47S.
[Google Scholar]
|
| 53. |
Lassen MR, Raskob GE, Gallus A, Pineo G, Chen D, Portman RJ. Apixaban or enoxaparin for thromboprophylaxis after knee replacement. N Engl J Med 2009;361:594-604.
[Google Scholar]
|
| 54. |
Lassen MR, Raskob GE, Gallus A, Pineo G, Chen D, Hornick P; ADVANCE – Investigators. Apixaban versus enoxaparin for thromboprophylaxis after knee replacement (ADVANCE-2): A randomised double-blind trial. Lancet 2010;375:807-15.
[Google Scholar]
|
| 55. |
Lassen MR, Gallus A, Raskob GE, Pineo G, Chen D, Ramirez LM; ADVANCE – Investigators. Apixaban versus enoxaparin for thromboprophylaxis after hip replacement. N Engl J Med 2010;363:2487-98.
[Google Scholar]
|
| 56. |
Friedman RJ, Dahl OE, Rosencher N, Caprini JA, Kurth AA, Francis CW, et al. Dabigatran versus enoxaparin for prevention of venous thromboembolism after hip or knee arthroplasty: A pooled analysis of three trials. Thromb Res 2010;126:175-82.
[Google Scholar]
|
| 57. |
Kearon C, Akl EA. Duration of anticoagulant therapy for deep vein thrombosis and pulmonary embolism. Blood 2014;123:1794-801.
[Google Scholar]
|
| 58. |
Toubi E, Krause I, Fraser A, Lev S, Stojanovich L, Rovensky J, et al. Livedo reticularis is a marker for predicting multi-system thrombosis in antiphospholipid syndrome. Clin Exp Rheumatol 2005;23:499-504.
[Google Scholar]
|
| 59. |
Tajima C, Suzuki Y, Mizushima Y, Ichikawa Y. Clinical significance of immunoglobulin A antiphospholipid antibodies: Possible association with skin manifestations and small vessel vasculitis. J Rheumatol 1998;25:1730-6.
[Google Scholar]
|
| 60. |
Dhir V, Pinto B. Antiphospholipid syndrome: A review. J Mahatma Gandhi Inst Med Sci 2014;19:19-27.
[Google Scholar]
|
| 61. |
Lateef A, Petri M. Clinical aspects of the antiphospholipid syndrome. In: Wallace DJ, Hahn BH, editors. Dubois' Lupus Erythematosus and Related Syndromes. 8th ed. Philadelphia: Elsevier Saunders; 2013. p. 518-25.
[Google Scholar]
|
| 62. |
Chighizola CB, Ubiali T, Meroni PL. Treatment of Thrombotic antiphospholipid syndrome: The rationale of current management – An insight into future approaches. J Immunol Res 2015;2015:951424.
[Google Scholar]
|
| 63. |
Erkan D, Harrison MJ, Levy R, Peterson M, Petri M, Sammaritano L, et al. Aspirin for primary thrombosis prevention in the antiphospholipid syndrome: A randomized, double-blind, placebo-controlled trial in asymptomatic antiphospholipid antibody-positive individuals. Arthritis Rheum 2007;56:2382-91.
[Google Scholar]
|
| 64. |
Girón-González JA, García del Río E, Rodríguez C, Rodríguez-Martorell J, Serrano A. Antiphospholipid syndrome and asymptomatic carriers of antiphospholipid antibody: Prospective analysis of 404 individuals. J Rheumatol 2004;31:1560-7.
[Google Scholar]
|
| 65. |
Ginsburg KS, Liang MH, Newcomer L, Goldhaber SZ, Schur PH, Hennekens CH, et al. Anticardiolipin antibodies and the risk for ischemic stroke and venous thrombosis. Ann Intern Med 1992;117:997-1002.
[Google Scholar]
|
| 66. |
Cervera R. Therapeutic strategies in antiphospholipid syndrome. Reumatol Clin 2010;6:37-42.
[Google Scholar]
|
| 67. |
Cuadrado MJ, Bertolaccini ML, Seed PT, Tektonidou MG, Aguirre A, Mico L, et al. Low-dose aspirin vs. low-dose aspirin plus low-intensity warfarin in thromboprophylaxis: A prospective, multicentre, randomized, open, controlled trial in patients positive for antiphospholipid antibodies (ALIWAPAS). Rheumatology (Oxford) 2014;53:275-84.
[Google Scholar]
|
| 68. |
Crowther MA, Ginsberg JS, Julian J, Denburg J, Hirsh J, Douketis J, et al. Acomparison of two intensities of warfarin for the prevention of recurrent thrombosis in patients with the antiphospholipid antibody syndrome. N Engl J Med 2003;349:1133-8.
[Google Scholar]
|
| 69. |
Finazzi G, Marchioli R, Brancaccio V, Schinco P, Wisloff F, Musial J, et al. Arandomized clinical trial of high-intensity warfarin vs. conventional antithrombotic therapy for the prevention of recurrent thrombosis in patients with the antiphospholipid syndrome (WAPS). J Thromb Haemost 2005;3:848-53.
[Google Scholar]
|
| 70. |
Ruiz-Irastorza G, Hunt BJ, Khamashta MA. A systematic review of secondary thromboprophylaxis in patients with antiphospholipid antibodies. Arthritis Rheum 2007;57:1487-95.
[Google Scholar]
|
| 71. |
Ruiz-Irastorza G, Crowther M, Branch W, Khamashta MA. Antiphospholipid syndrome. Lancet 2010;376:1498-509.
[Google Scholar]
|
| 72. |
Coloma Bazán E, Donate López C, Moreno Lozano P, Cervera R, Espinosa G. Discontinuation of anticoagulation or antiaggregation treatment may be safe in patients with primary antiphospholipid syndrome when antiphospholipid antibodies became persistently negative. Immunol Res 2013;56:358-61.
[Google Scholar]
|
| 73. |
Criado-García J, Fernández-Puebla RA, Jiménez LL, Velasco F, Santamaría M, Blanco-Molina A. Anticoagulation treatment withdrawal in primary antiphospholipid syndrome when anticardiolipin antibodies become negative. Rev Clin Esp 2008;208:135-7.
[Google Scholar]
|
| 74. |
Fonseca AG, D'Cruz DP. Controversies in the antiphospholipid syndrome: Can we ever stop warfarin? J Autoimmune Dis 2008;5:6.
[Google Scholar]
|
| 75. |
Vargas-Hitos JA, Ateka-Barrutia O, Sangle S, Khamashta MA. Efficacy and safety of long-term low molecular weight heparin in patients with antiphospholipid syndrome. Ann Rheum Dis 2011;70:1652-4.
[Google Scholar]
|
| 76. |
Bick RL, Rice J. Long-term outpatient dalteparin (fragmin) therapy for arterial and venous thrombosis: Efficacy and safety – A preliminary report. Clin Appl Thromb Hemost 1999;5 Suppl 1:S67-71.
[Google Scholar]
|
| 77. |
Empson M, Lassere M, Craig JC, Scott JR. Recurrent pregnancy loss with antiphospholipid antibody: A systematic review of therapeutic trials. Obstet Gynecol 2002;99:135-44.
[Google Scholar]
|
| 78. |
Bittar M, Uthman I. Antiphospholipid syndrome novel therapies. EMJ Rheumatol 2014;1:113-21.
[Google Scholar]
|
| 79. |
Noel N, Dutasta F, Costedoat-Chalumeau N, Bienvenu B, Mariette X, Geffray L, et al. Safety and efficacy of oral direct inhibitors of thrombin and factor Xa in antiphospholipid syndrome. Autoimmun Rev 2015;14:680-5.
[Google Scholar]
|
| 80. |
Son M, Wypasek E, Celinska-Lowenhoff M, Undas A. The use of rivaroxaban in patients with antiphospholipid syndrome: A series of 12 cases. Thromb Res 2015;135:1035-6.
[Google Scholar]
|
| 81. |
Cohen H, Doré CJ, Clawson S, Hunt BJ, Isenberg D, Khamashta M, et al. Rivaroxaban in antiphospholipid syndrome (RAPS) protocol: A prospective, randomized controlled phase II/III clinical trial of rivaroxaban versus warfarin in patients with thrombotic antiphospholipid syndrome, with or without SLE. Lupus 2015;24:1087-94.
[Google Scholar]
|
| 82. |
Rivaroxaban in Thrombotic Antiphospholipid Syndrome (TRAPS). Available from: https://www.clinicaltrials.gov/ct2/show/NCT02157272. [Last accessed on 2015 Jul 29].
[Google Scholar]
|
| 83. |
Rivaroxaban for Antiphospholipid Antibody Syndrome (RAPS). Available from: https://www.clinicaltrials.gov/ct2/show/NCT02116036. [Last accessed on 2015 Jul 29].
[Google Scholar]
|
| 84. |
Apixaban for the Secondary Prevention of Thromboembolism among Patients with the Antiphospholipid Syndrome (ASTRO-APS). Available from: https://www.clinicaltrials.gov/ct2/show/NCT02295475. [Last accessed on 2015 Jul 29].
[Google Scholar]
|
| 85. |
Denton CP, Howell K, Stratton RJ, Black CM. Long-term low molecular weight heparin therapy for severe Raynaud's phenomenon: A pilot study. Clin Exp Rheumatol 2000;18:499-502.
[Google Scholar]
|
| 86. |
Balbir-Gurman A, Markovits D, Nahir AM, Rozin A, Braun-Moscovici Y. Ischemic finger ulcer as a presenting symptom of systemic sclerosis. Isr Med Assoc J 2005;7:531-2.
[Google Scholar]
|
| 87. |
Francès C, Papo T, Wechsler B, Laporte JL, Biousse V, Piette JC. Sneddon syndrome with or without antiphospholipid antibodies. A comparative study in 46 patients. Medicine (Baltimore) 1999;78:209-19.
[Google Scholar]
|
| 88. |
Wu S, Xu Z, Liang H. Sneddon's syndrome: A comprehensive review of the literature. Orphanet J Rare Dis 2014;9:215.
[Google Scholar]
|
| 89. |
Theodoridis A, Makrantonaki E, Zouboulis CC. Malignant atrophic papulosis (Köhlmeier-Degos disease) – A review. Orphanet J Rare Dis 2013;8:10.
[Google Scholar]
|
| 90. |
Cox NH, Piette WW. Purpura and microvascular occlusion. In: Burns T, Breathnach S, Cox N, Griffiths C, editors. Rook's Textbook of Dermatology. 8th ed. Oxford: Wiley-Blackwell; 2010. p. 49.1-49.51.
[Google Scholar]
|
| 91. |
Stefanidou MP, Ioannidou DJ, Panayiotides JG, Tosca AD. Low molecular weight heparin; a novel alternative therapeutic approach for lichen planus. Br J Dermatol 1999;141:1040-5.
[Google Scholar]
|
| 92. |
Ferahbas A, Uksal U, Kutlugun C, Kontas O. Low-molecular-weight heparin (enoxaparin) in the treatment of lichen planus. J Eur Acad Dermatol Venereol 2003;17:604-5.
[Google Scholar]
|
| 93. |
Uçmak D, Balcı G, Harman M. The effectiveness of treatment with enoxaparin in lichen planus. J Clin Exp Invest 2011;3:172-3.
[Google Scholar]
|
| 94. |
Iraji F, Asilian A, Saeidi A, Siadat AH, Saeidi AR, Hassanzadeh A. Comparison of therapeutic effect of low-dose low-molecular-weight heparin (enoxaparin) vs. oral prednisone in treatment of patients with lichen planus; A clinical trial. Adv Biomed Res 2013;2:76.
[Google Scholar]
|
| 95. |
Ameen WA, Alfadhily ZS. Treatment of recalcitrant lichen planus with low molecular weight heparin (Enoxaparin): A case series study among Iraqi patients. Med J Babylon 2011;8:93-103.
[Google Scholar]
|
| 96. |
Neville JA, Hancox JG, Williford PM, Yosipovitch G. Treatment of severe cutaneous ulcerative lichen planus with low molecular weight heparin in a patient with hepatitis C. Cutis 2007;79:37-40.
[Google Scholar]
|
| 97. |
Murao K, Kubo Y. A case of lichen planus significantly improved after topical heparinoid treatment. Australas J Dermatol 2013;54:236-7.
[Google Scholar]
|
| 98. |
Cholongitas E, Kokolakis G, Giannikaki E, Ioannidou D. Persistent generalized lichen nitidus successfully treated with enoxaparin sodium. Am J Clin Dermatol 2008;9:349-50.
[Google Scholar]
|
| 99. |
Ghaffari S, Barikbin L, Ashnagar S, Hajji Fattahi F, Tavakoli Kia R, Rezaiee M. Enoxaparin for the treatment of recurrent aphthous stomatitis: A pilot exploratory clinical trial. Minerva Stomatol 2013;62:281-7.
[Google Scholar]
|
| 100. |
Femiano F, Gombos F, Scully C. Recurrent aphthous stomatitis unresponsive to topical corticosteroids: A study of the comparative therapeutic effects of systemic prednisone and systemic sulodexide. Int J Dermatol 2003;42:394-7.
[Google Scholar]
|
| 101. |
Cugno M, Tedeschi A, Crosti C, Marzano AV. Activation of blood coagulation in autoimmune skin disorders. Expert Rev Clin Immunol 2009;5:605-13.
[Google Scholar]
|
| 102. |
Asero R, Tedeschi A, Cugno M. Heparin and tranexamic acid therapy may be effective in treatment-resistant chronic urticaria with elevated d-dimer: A pilot study. Int Arch Allergy Immunol 2010;152:384-9.
[Google Scholar]
|
| 103. |
Shah SA, Ormerod AD. Dermatitis herpetiformis effectively treated with heparin, tetracycline and nicotinamide. Clin Exp Dermatol 2000;25:204-5.
[Google Scholar]
|
| 104. |
Tan CC, Sale JE, Brammer C, Irons RP, Freeman JG. A rare case of dermatitis herpetiformis requiring parenteral heparin for long-term control. Dermatology 1996;192:185-6.
[Google Scholar]
|
| 105. |
Kachewar S, Singh BH, Sasane AG, Bhadane S. Full blown case of lipoid proteinosis. MJAFI 2011;67:90-1.
[Google Scholar]
|
| 106. |
Aşıcıoğlu E, Kahvecİ A, Koç M, Özener C. Uremic pruritus: Still itching. Turk Neph Dial Transpl 2011;20:7-13.
[Google Scholar]
|
| 107. |
James WD, Berger TG, Elston DM, editors. Contact dermatitis and drug eruptions. In: Andrews' Diseases of the Skin Clinical Dermatology. 11th ed. Philadelphia: Elsevier Saunders; 2011. p. 88-137.
[Google Scholar]
|
| 108. |
Kakagia DD, Papanas N, Karadimas E, Polychronidis A. Warfarin-induced skin necrosis. Ann Dermatol 2014;26:96-8.
[Google Scholar]
|
| 109. |
Choudhary SV, Madnani A, Singh AL. Late onset warfarin induced skin necrosis in human immunodeficiency virus infected patient with pulmonary tuberculosis. Indian J Sex Transm Dis 2013;34:47-9.
[Google Scholar]
|
| 110. |
Xin C, Hu D, Li M. Late onset warfarin-induced skin necrosis, a case report. G Ital Dermatol Venereol 2014; [Epub ahead of print].
[Google Scholar]
|
| 111. |
McGevna LF, Raugi GJ, Raza SR. Cutaneous Manifestation of Cholesterol Embolism Clinical Presentation. Available from: http://www.emedicine.medscape.com/article/1096593-clinical. [Last accessed on 2015 Aug 04].
[Google Scholar]
|
| 112. |
McGevna LF, Raugi GJ, Raza SR. Cutaneous Manifestation of Cholesterol Embolism Treatment and Management. Available from: http://www.emedicine.medscape.com/article/1096593-treatment. [Last accessed on 2015 Aug 04].
[Google Scholar]
|
| 113. |
Piette WW. Cutaneous manifestations of microvascular occlusion syndromes. In: Bolognia JL, Jorizzo JL, Schaffer JV, editors. Dermatology. 3rd ed. London: Elsevier; 2008. p. 369-84.
[Google Scholar]
|
| 114. |
Krzych LJ, Nowacka E, Knapik P. Heparin-induced thrombocytopenia. Anaesthesiol Intensive Ther 2015;47:63-76.
[Google Scholar]
|
| 115. |
Al-Eidan FA. Is the incidence trend of heparin-induced thrombocytopenia decreased by the increased use of low-molecular-weight-heparin? Mediterr J Hematol Infect Dis 2015;7:e2015029.
[Google Scholar]
|
| 116. |
Lewis BE, Wallis DE, Berkowitz SD, Matthai WH, Fareed J, Walenga JM, et al. Argatroban anticoagulant therapy in patients with heparin-induced thrombocytopenia. Circulation 2001;103:1838-43.
[Google Scholar]
|
| 117. |
Joseph L, Casanegra AI, Dhariwal M, Smith MA, Raju MG, Militello MA, et al. Bivalirudin for the treatment of patients with confirmed or suspected heparin-induced thrombocytopenia. J Thromb Haemost 2014;12:1044-53.
[Google Scholar]
|
| 118. |
Warkentin TE, Greinacher A, Koster A, Lincoff AM; American College of Chest Physicians. Treatment and prevention of heparin-induced thrombocytopenia: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition). Chest 2008;133 6 Suppl: 340S-80S.
[Google Scholar]
|
| 119. |
Breathnach SM. Drug reactions. In: Burns T, Breathnach S, Cox N, Griffiths C, editors. Rook's Textbook of Dermatology. 8th ed. Oxford: Wiley-Blackwell; 2010. p. 75.1-75.117.
[Google Scholar]
|
| 120. |
Issa AA, Simman R. Lovenox induced tissue necrosis, a case report and literature review. J Am Coll Clin Wound Spec 2015;5:66-8.
[Google Scholar]
|
| 121. |
Fatma LB, El Ati Z, Azzouz H, Rais L, Krid M, Smaoui W, et al. Subcutis calcinosis caused by injection of calcium-containing heparin in a chronic kidney injury patient. Saudi J Kidney Dis Transpl 2014;25:1068-71.
[Google Scholar]
|
| 122. |
Nuno-Gonzalez A, Calzado-Villarreal L, Gutierrez-Pascual M, Gamo-Villegas R, Sanz-Robles H, Sanchez-Gilo A, et al. An unusual adverse effect of nadroparin injections: Calcinosis cutis. Dermatol Online J 2011;17:4.
[Google Scholar]
|
| 123. |
Smith KJ, Rosario-Collazo J, Skelton H. Delayed cutaneous hypersensitivity reactions to hirudin. Arch Pathol Lab Med 2001;125:1585-7.
[Google Scholar]
|
| 124. |
Rebora A. Telogen effluvium revisited. G Ital Dermatol Venereol 2014;149:47-54.
[Google Scholar]
|
| 125. |
Wang YY, Po HL. Enoxaparin-induced alopecia in patients with cerebral venous thrombosis. J Clin Pharm Ther 2006;31:513-7.
[Google Scholar]
|
| 126. |
Sharif S, Hay CR, Clayton-Smith J. Aplasia cutis congenita and low molecular weight heparin. BJOG 2005;112:256-8.
[Google Scholar]
|
| 127. |
Brown DG, Wilkerson EC, Love WE. A review of traditional and novel oral anticoagulant and antiplatelet therapy for dermatologists and dermatologic surgeons. J Am Acad Dermatol 2015;72:524-34.
[Google Scholar]
|
| 128. |
Plovanich M, Mostaghimi A. Novel oral anticoagulants: What dermatologists need to know. J Am Acad Dermatol 2015;72:535-40.
[Google Scholar]
|
Fulltext Views
12,508
PDF downloads
3,227





