Translate this page into:
Alopecia areata: An update
Correspondence Address:
Kolalapudi Anjaneyulu Seetharam
3-28-18/155, Rajendranagar 4th line, Guntur - 522 006, AP
India
| How to cite this article: Seetharam KA. Alopecia areata: An update. Indian J Dermatol Venereol Leprol 2013;79:563-575 |
Abstract
Alopecia areata (AA) is a common form of non-scarring hair loss of scalp and/or body. Genetic predisposition, autoimmunity, and environmental factors play a major role in the etiopathogenesis of AA. Patchy AA is the most common form. Atopy and autoimmune thyroiditis are most common associated conditions. Peribulbar and intrabulbar lymphocytic inflammatory infiltrate resembling "swarm of bees" is characteristic on histopathology. Treatment is mainly focused to contain the disease activity. Corticosteroids are the preferred treatments in form of topical, intralesional, or systemic therapy. Camouflage in the form of wigs may be an alternative option in refractory cases.Introduction
Alopecia areata (AA) is a common form of non-scarring alopecia involving the scalp and/or body, characterized by hair loss without any clinical inflammatory signs. It is one of the most common form of hair loss seen by dermatologists and accounts for 25% of all the alopecia cases. [1] It was first described by Cornelius Celsus, and the term AA was coined by Sauvages in 1760. [2] It accounts for 2-3% of the new dermatology cases in UK and USA, 3.8% in China, and 0.7% in India. [2],[3],[4] In general population, the prevalence was estimated at 0.1-0.2% with a lifetime risk of 1.7%. [4] Both males and females are equally affected, [5] but some studies reported male preponderance. [2],[4],[6],[7] It can occur at any age. The youngest was 4-months-old, and the oldest was in late seventies. [8] Twenty percent of cases were children, and 60% of AA patients had their first patch before 20 years of age. [5] Highest prevalence was between 30-59 yrs of age. [1] Family members are affected in 8.7-20% of cases. [2],[8]
Etiopathogenesis
Hair growth and maintenance depends on 3 phases of hair cycle, anagen (active growth phase), catagen (involution phase), and telogen (resting phase). The type and length of the hair depends on the anagen phase. In normal healthy individuals, hair sheds out after the resting phase when the new hair anagen growth starts (exogen). In alopecias, hair shedding occurs even before the anagen starts leaving the hair follicle empty (kenogen). Thus, AA is generally a disorder of hair cycling and is considered to be a state of kenogen. [9]
The etiology of AA is still an enigma. Many hypotheses are proposed. Epidemics of AA reported from orphanages and schools pointed towards infectious etiology. [5] Viral etiology was proposed initially, but the later research did not confirm that. [10] AA occurring in monozygotic twins and a strong family history for many generations in families of AA individuals shows that AA can be inherited. [9],[11] In AA, 4-28% had one affected family member, and polygenic inheritance was suggested. [12] Functional gene alleles, that code towards autoimmunity and specially towards AA, are described in AA individuals. Candidate gene studies showed an association to genes in HLA region (HLA-DQB1, HLA-DRB1, HLA-A, HLA-B, HLA-C, NOTCH4, MICA) as well as genes outside HLA. The HLA-DQB1FNx01 03 allele, among others, may be an important marker for susceptibility to the disease. [13],[14] Genome wide scan confirmed the link between AA and MHC region on chromosome 6p and identified susceptible loci on chromosomes 10, 16, and 18. [12] Recently, genome wide association studies (GWAS) identified specific genetic markers for AA. GWAS can recognize specific individual genes, which may increase the risk for AA. Petukhova et al. surveyed the entire genome and identified 139 single nucleotide polymorphisms (SNPs) for AA, clustered in 8 regions of the genome. [13],[15] GWAS studies had found key genes in AA related to T-cells (IL2/IL21, IL2RA, CTLA4, IKZF4, HLA) and hair follicle (NK-activating ligands-ULBP3, ULBP6, STX17, PRDX5). [15] All these point towards genetic predisposition in the development of AA.
Besides genetic susceptibility, various triggering factors like stress, hormones, diet, infectious agents, vaccinations and many others were incriminated in the pathogenesis of AA. [16],[17] Stress is considered as one of the triggers, but controlled studies did not confirm this. [2],[17],[18] Emotional trauma of a family death or an accident have been reported as precipitating factors in individual cases, but there are no controlled studies proving this. Iron deficiency was noted in 24-71% of females with AA. [19] AA was less frequently observed in people, taking diet rich in soy oil. [20] Cytomegalovirus infections and hepatitis B vaccination were implicated, but further studies failed to confirm any correlation. [10],[21] Some studies found decreased levels of zinc in the blood of AA patients, [7] and others reported conflicting results. [22] Roselino et al. reported an outbreak of AA in workers at a water treatment plant in a paper factory and was linked to long-term exposure to the chemical acrylamide. [23]
Recently, AA is considered as an autoimmune disease. The association with other autoimmune diseases like thyroid disease, anemia, diabetes mellitus, vitiligo, and psoriasis may be one of the causes to believe AA is an autoimmune disease. [24],[25] Hair follicle-specific antibodies are increased in peripheral blood of AA patients, especially to keratin 16 and trichohyalin. [26] It is believed that hair follicle is an immune-privileged site. [9] In healthy hair follicle epithelium, major histocompatibility complex (MHC) class I and II molecules are not expressed and TGF- β,IGF-1, and α-MSH are more expressed. [27] This immune privilege is collapsed in AA by the presence of increased MHC I and II complexes, decreased immunosuppressive molecules, and higher expression of adhesion molecules (ICAM-2 and ELAM-1) in the perivascular and peribulbar hair follicular epithelium, leading to perifollicular inflammation. [28] This peribulbar inflammation adversely affects hair follicle activity, resulting in thin dystrophic hair with miniaturization. [16] Thus, AA is considered as hair follicle-specific autoimmune disease, triggered by environmental factor in genetically susceptible individuals. [9],[16]
Clinical Features
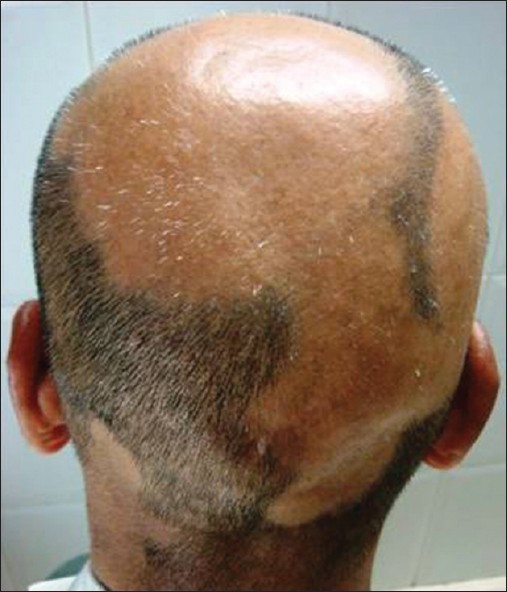
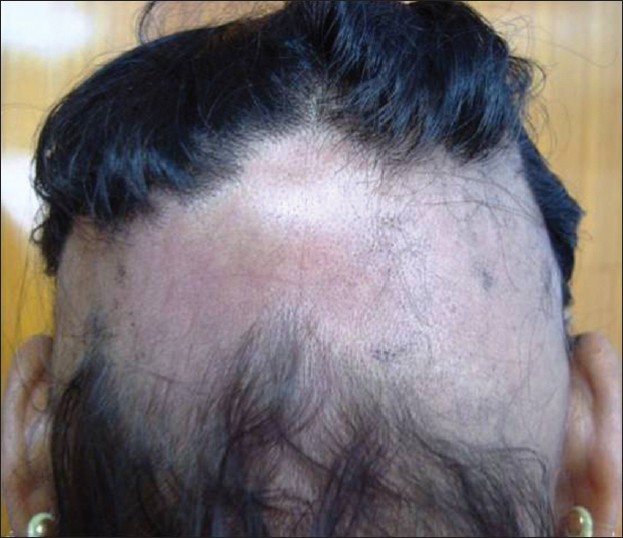
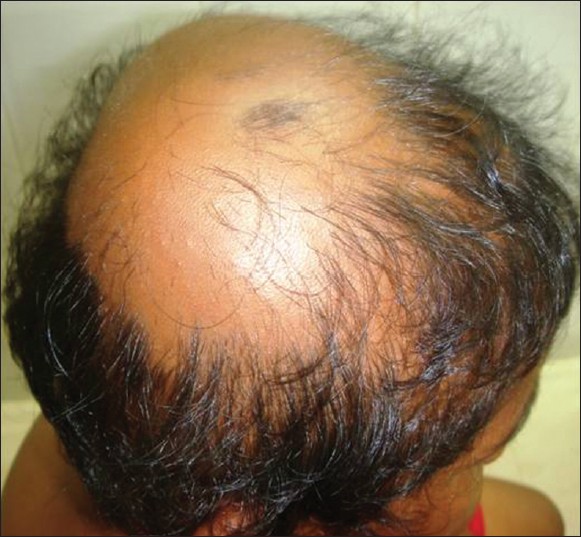
AA commonly manifests as localized, well-demarcated patches of hair loss. Often, they are suddenly noticed, and they may progress circumferentially. It may present as single or multiple patches [Figure - 1]. Small distinct patches may merge and form larger patches [Figure - 2]. Scalp is the most common site (90%), but any part of the body may be affected. AA can be classified depending on extent and pattern of hair loss [29] [Table - 1]. It can be patchy AA, alopecia totalis (AT) involving the entire scalp and body hair such as eyebrows, eyelashes, beard, axillary hair and pubic hair and alopecia universalis (AU) if the total body hair is involved. [5] 5-10% of patchy AA may progress to AT/AU. If AA develops before puberty, the risk of AT is 50% and in older individuals, the risk is about 25%. [10],[30] The pattern of hair loss can be reticular, ophiasis, and sisaipho. Ophiasis (snake-like) is a band-like AA along the posterior occipital and temporal margins [Figure - 3]. Sisaipho, also called as ophiasis inversus, presents with alopecia involving the frontal, temporal, and parietal scalp but spares hair along the scalp periphery, mimicking androgenetic alopecia [Figure - 4]. Acute diffuse and total alopecia, a new variant, was recently described. [31],[32] It is characterized by female preponderance, generalized thinning, rapid progression, tissue eosinophilia, extensive involvement, brief clinical course, and favorable prognosis. Yesudian et al. reported another unusual variant of AA, perinevoid alopecia, alopecia patches around the nevi. [33] Sometimes, unusual presentations may occur in linear distribution [Figure - 5].
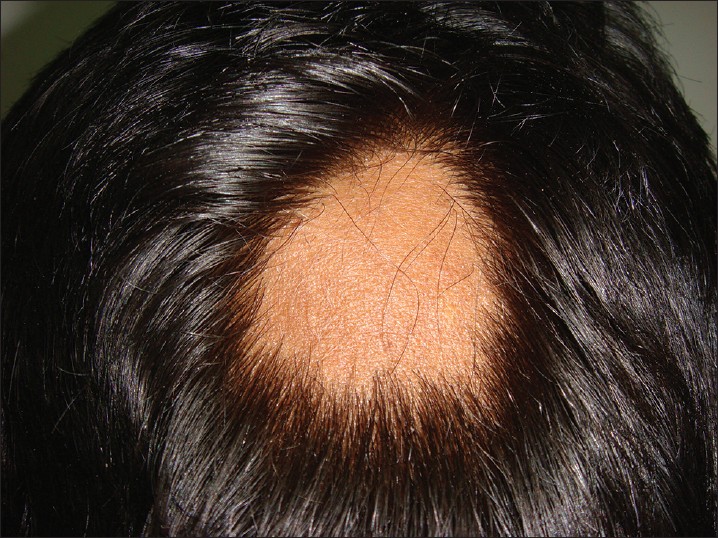 |
| Figure 1: Localized patch of alopecia areata |
 |
| Figure 2: Small patches, merging and forming larger patch |
 |
| Figure 3: Ophiasis |
 |
| Figure 4: Sisaipho |
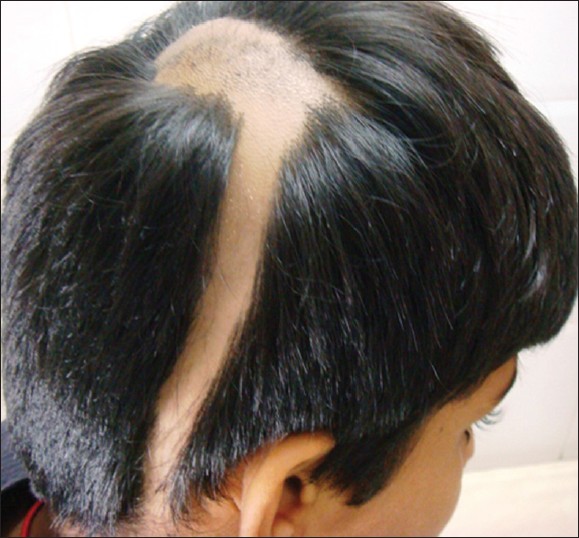 |
| Figure 5: Unusual presentation of alopecia areata in a linear pattern |

Ikeda classified AA based on the associated conditions and on the course of the disease. [34]
Atopic type: It begins early in life and mostly (30-75%) progresses to AT.
Autoimmune type: It is seen in middle-aged groups associated with autoimmune diseases, diabetes mellitus and progresses to AT in 10-50%.
Prehypertensive type: It is seen in young adults whose parents were hypertensive and progress fastly to AT in 40% of cases.
Common type: It affects adults aged 20-40 years and AT develops in 5-15% of cases.
Typically, the surface of AA patches is smooth and normal skin color without any skin alterations like scaling and follicular changes. Rarely, it can be peachy or red. [29] Characteristic ′exclamatory mark hairs′ are seen either within or at the border of the patches. These are fractured and short hairs with proximal tapering, close to scalp and distal thickening and widening. [5] The presence of exclamatory hairs at the border and the hair pull test with 6 or more hairs from the periphery suggests that the patch may be active and progressive. [5],[29] Shuster described coudability hairs (A kink in the normal looking hairs, at a distance of 5-10 mm above the surface, when the hair was bent inwards) in patchy AA. [35] Initially, white hairs are spared involving only pigmented hair causing sudden whitening of hair (canites subita); however, in chronic cases, the white hair is also lost. [36] Mostly, AA patients are asymptomatic, and rarely pruritus, pain and/or burning sensation may precede hair loss. [29] The diagnosis is mostly clinical and does not cause difficulty many times. Dermoscopy is useful in doubtful cases.
Dermoscopy
Dermoscopy is an easy and useful technique to observe hair loss. Dry dermoscopy, also called trichoscopy, is ideal because it has the blocking filter against light reflection from the skin surface and it can be done directly without application of the gel. [37] Characteristic dermoscopic features of AA are yellow dots, black dots, broken hairs, tapering hair (exclamation marks), and short vellus hairs [38],[39],[40],[41] [Figure - 6]. Inui et al. described coudability hairs on trichoscopy and suggested that they are useful markers for disease activity in AA. [41] Presence of black dots, broken hair, and tapering hair suggest active disease. Black dots and yellow dots are proportional to severity of AA, and tapering hair does not have any correlation with severity. [40] Yellow dots are also seen in androgenetic alopecia, but they are few in number, whereas in AA, they are abundant. Single feature on dermoscopy may not be diagnostic of AA, but a combination of features are helpful in detecting difficult cases like AA incognito. [39],[40]
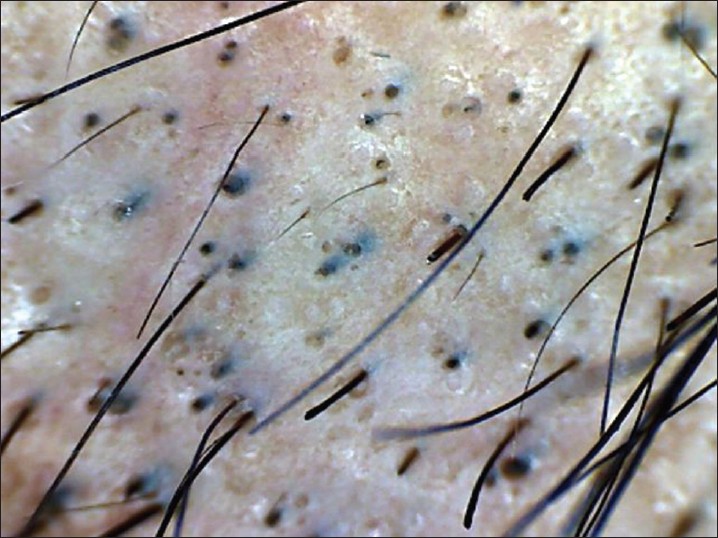 |
| Figure 6: Dermoscopy of AA, showing-yellow dots, black dots, broken hair and tapering hair |
Nail Changes
Nail changes are seen in 29% of adults and 50% of children with AA. They are more common in males and severe AA. [42],[43],[44] Nail changes may precede or follow the hair loss, and they may be limited to one or most nails. Nail changes typical of AA are geometric pitting (multiple, small, superficial pits regularly distributed along transverse and longitudinal lines), geometric punctate leukonychia (multiple white spots in a grill pattern), and trachyonychia (sandpaper nails). [45] The other changes include Beau′s lines, onychomadesis, red lunulae, and red lunulae indicate acute and severe disease.
Associated Conditions
AA is associated with atopy in 10-22%, twice the prevalence in general population. [46]
Autoimmune thyroiditis is associated with 8-28% of cases, and thyroid antibodies do not have any clinical correlation with severity. [47] It is less common in Japan and Netherlands. [2] In India, an earlier study by Sharma et al. [2] reported autoimmune thyroiditis in only 1% of AA patients, whereas a recent study showed thyroiditis in 18.3% of AA cases. [48] The other associated conditions are vitiligo, psoriasis, diabetes mellitus, Down′s syndrome, Addison′s disease, autosomal recessive autoimmune polyglandular syndrome, systemic lupus erythematosus, celiac disease, ulcerative colitis, and multiple sclerosis. These are less common and are more likely to be associated with AT/AU. [49] Sharma et al. reported that presence of vitiligo in family embers was a definite risk factor for developing severe forms of alopecia. [2] The family members of AA have increased incidence of type I diabetes, whereas the AA patients themselves have reduced incidence. [50] In a recent nationwide cohort study from Taiwan, Chu et al. described the association of these co-morbidities on the basis of age of AA onset. [51] Patients presenting with AA in childhood (<10 yrs of age) are most likely to have atopic dermatitis or SLE, patients in the second decade have high risk for psoriasis or rheumatoid arthritis, and patients presenting with AA in the old age (>60 years) are more likely to have thyroid disease. These observations are giving clues about screening tests to be ordered in various age groups of patients with AA. Anxiety and mood disturbances are frequently present with AA and may result in reduced self-esteem and may have a negative impact on quality of life (QOL). [6],[52] Punctate lens opacities, early cataracts, and fundus abnormalities may occur in 40% to 50% of patients with AA. [53]
Histopathology
The histology findings in AA vary with the duration of disease. It is ideal to perform two 4 mm punch biopsies including subcutaneous fat. One specimen should be processed with vertical sectioning and the other with horizontal sectioning. If only a single specimen is planned, horizontal sections will give a better representation of the histopathology. A horizontally-sectioned scalp biopsy is helpful in confirming the diagnosis of AA but also provides information about possible regrowth. [54] However, Chaitra et al. reported that vertical ections are adequate to ascertain the diagnosis. [55] The best place to take a biopsy is at the advancing border of hair loss. This helps to view the hair follicles at different levels in dermis to quantify the hair follicle density, follicle diameter, and to assess the proportion of hair follicles in various stages. A mean count of less than one follicle/mm 2 usually indicates less chances of regrowth. [54]
In acute cases, peribulbar and intrabulbar lymphocytic inflammatory infiltrate around anagen follicles, resembling ′swarm of bees,′ is characteristic. The lymphocytes are mainly around the hair matrix and dermal papilla and spare the bulge area, causing follicular edema, cellular necrosis, microvesiculation, and pigment incontinence. A dense lymphocytic inflammation can cause weakening of the hair shaft resulting in a trichorrhexis nodosa-like fracture, leading to the exclamation mark hairs. [56] In subacute lesions, high proportion of catagen/telogen hair follicles are seen. In chronic cases, follicular miniaturization with variable inflammatory infiltrate are seen in papillary dermis. The terminal to vellus hair ratio is decreased to 1:1 in contrast to 7:1 in normal population. Androgenetic alopecia also shows follicular miniaturization, but more number of telogen hairs with decreased anagen to telogen ratio may be a clue towards AA. [54]
Differential Diagnosis
It is often easy and simple to detect AA. Many conditions may mimic AA [Table - 2]. Tinea capitis, especially in children, should be differentiated. Signs of inflammation, scaling, and cervical lymphadenopathy are present in tinea capitis, in contrast to smooth, non-scaly surface of AA. Trichotillomania presents with broken hair of varying lengths with a wire brush feel compared to smooth hair loss of AA. Cicatricial alopecia is characterized by patchy hair loss with loss of follicular orifices. Erythema, scaling, pustulation, and plugging may occur based on the underlying cause. Androgenetic alopecia is gradual hair loss with patterned distribution. Diffuse AA may resemble androgenetic alopecia and telogen effluvium. The progression is rapid and widespread in diffuse AA. In doubtful cases, a scalp biopsy may be of help. Side pins, which are used by women to keep the hair in place, may cause pressure alopecia, resembling AA [Figure - 7]a and b. Traction alopecia is another condition, which mimics AA. Secondary syphilis produces moth-eaten alopecia rather than smooth surface of AA. Congenital triangular alopecia closely mimics AA. It is not congenital as the name suggests, appear usually after 2 years of age, rarely in adulthood also. Scalp biopsy is needed to identify, which shows normal number of hair follicles, but all are vellus or indeterminate. [57]
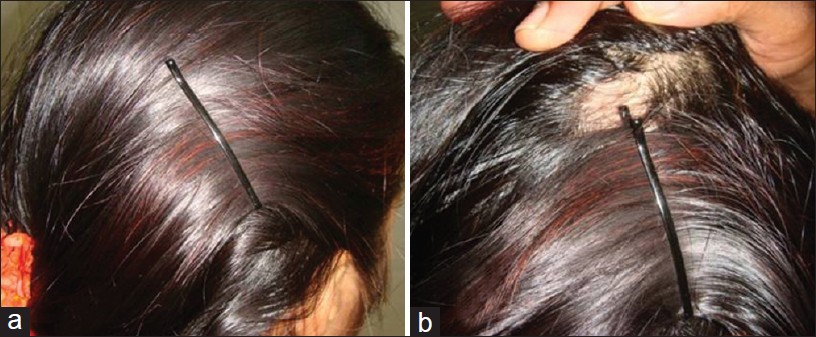 |
| Figure 7: (a and b) Sidepin alopecia |

Investigations
Most of the AA cases are typical and obvious; therefore, laboratory tests are not necessary. Thyroid screening is not mandatory as thyroid disease and AA are not correlated clinically or causally. [9] Thyroid screening may be of use in long-standing cases, females with persistent patches, patients with suggestive symptoms of thyroid disease and severe AA (AT/AU). Potassium hydroxide smear, fungal culture, serology for syphilis, and scalp biopsy may help in doubtful cases. [9] Hair pull test, hair pluck test, dermoscopy, SALT score (severity of alopecia tool score) are useful in assessing the activity and severity of the disease. [58] Optical coherence tomography (OCT) is a recently evaluated non-invasive technique to detect the hair shaft abnormalities in AA. Bartles et al. demonstrated that the cross section of hairs from an AA patch was significantly lower compared with hairs of an unaffected area by this OCT. [59] Thus, OCT may be an useful non-invasive technique to differentiate AA from other causes of patchy alopecia, such as trichotillomania. Presence of exclamatory mark hairs at periphery, positive hair pull test (>6 hairs), daily hair count (>100 hairs), hair pluck test (more telogen hairs) and dermoscopy (black dots, brokenhair, and tapering hair) suggest active disease. Severity of AA can be measured by SALT score, developed by the National Alopecia Areata Foundation working committee. [60]
SALT Score
SALT score is useful to find out the quantitative assessment of scalp hair loss. [60] The entire scalp was divided into 4 parts based on the surface area, top (40% - 0.4), posterior (24% - 0.24), right side (18% - 0.18), and left side of scalp (18% - 0.18). Percentage of hair loss in each area is determined independently and is multiplied by the percentage of scalp covered in that area of the scalp, and summing the products of each area will give the SALT score. For example, the hair loss is 40%, 30%, 20% and 10% in top, back and right and left side respectively, then the SALT score can be calculated as- (40 × 0.4) + (30 × 0.24) + (20 × 0.18) + (10 × 0.18) =16 + 7.2 + 3.6 + 1.8 = 28.6. SALT score is easily reproducible and validated. However, it does not include hair pigmentation, body hair, and nail involvement.
Course and Prognosis
Spontaneous regrowth occurs in many patients. Most will have more than one episode. 50-80% of patchy AA patients may regrow hair in one year. Few of them may persist for longer time, and some may never recover hair. [3],[9],[61] Some of the clinical features suggest poor prognosis [Table - 3]. [9],[29] The disease may progress and worsen in children, even with milder initial presentation. [61]

5-10% may progress to AT/AU. The chance of full recovery is less than 10% in AT/AU. [3]
Treatment
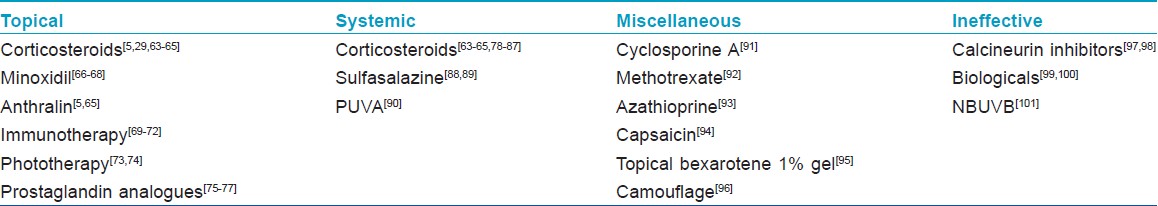
AA is mainly a cosmetic concern, causing more emotional problems, especially in children and women. Spontaneous remissions can occur in up to 80% of limited AA within one year. [34] A recent detailed Cochrane review revealed paucity of randomized controlled studies in documenting efficacious treatments for AA. [62] Definitive cure or preventive treatment was not established, and the focus of treatment is mainly towards curtailing the disease activity. Counseling and informing the possible true expectations of the available treatments are important. The documented treatments are mentioned in [Table - 4].
Corticosteroids
Corticosteroids, because of their anti-inflammatory activity, have been the mainstay of therapy for AA. They have been used topically, orally, and parenterally. Different forms of topical steroids are used with variable efficacy. Fluocinolone acetonide 0.2% cream, 0.1% betamethasone valerate foam, 0.05% betamethasone dipropionate lotion, 0.1% halcinonide, 0.05% clobetasol ointment/foam have been used with a success range of 28.5% - 61%. [63] It is recommended to use 1 cm beyond the involved area. Relapses were seen in 37.5% of the responders despite continuation of treatment. [63] Despite variable efficacy, topical steroids are preferred first choice in the treatment of AA because of ease of application, especially in children. Midpotent topical steroids are frequent choice in children. [64] Folliculitis, telangiectasia, and atrophy can occur. [64] They can be applied alternate day or 5 days a week to prevent atrophy. [65] Application under occlusion increases the potency of topical corticosteroids and in turn side effects.
Intralesional corticosteroids have been used since 1958 in the treatment of AA, and it is the treatment of choice for adults in patchy AA, with approximate success rates of 60-75%. [64] Triamcinolone acetonide is preferred. It should be injected into deep dermis or upper subcutaneous tissue using a 0.5-inch long 30-gauge needle at multiple sites, 1 cm apart and 0.1 ml into each site, once in 4-6 weeks. [29],[37] Various concentrations (2.5-10 mg/ml) are used, but 10 mg/ml is preferred for scalp and 2.5 mg/ml for eye brows and face. [29] The maximum dose should not exceed 20 mg for each visit. [78] Regrowth is usually visible in 4 weeks, and intralesional corticosteroids should be discontinued if there is no improvement in 6 months. Some are resistant to steroid therapy because of decreased expression of thioredoxin reductase 1, an enzyme that activates the glucocorticoid receptor in the outer root sheath. [79] Atrophy is commonly observed, and it can be minimized by avoiding superficial injections, minimizing the volume and concentrations and spacing the injection sites. [64] The atrophy is usually transient, and normal thickness of the skin is regained in course of time. Hypopigmentation, telangiectasia and rarely anaphylaxis are few more concerns. Cataract and raised intraocular pressure can occur if intralesional corticosteroids are used near the eyebrows.
Systemic corticosteroids have been used daily, weekly, and monthly pulses with good improvement in patchy AA and less favorable outcome in ophiasis, AT, and AU. [80],[81] Oral dexamethasone 0.5 mg/kg/day, intramuscular triamcinolone acetonide 40 mg/month have been tried successfully. [82] Steroids can be continued for 1-6 months, but prolonged periods are avoided, especially in children, because of side effects. [5] Pulse therapies are tried to avoid the side effects. Intravenous methylprednisolone and dexamethasone in pulse form have shown successful results. [80],[83] Oral pulse therapies are more successful and acceptable with lesser side effects. Kar et al. reported 60% success with 200 mg of prednisolone once-weekly for 3 months with a observational period for another 6 months. [81] Some other studies showed cosmetic regrowth in 58-82% of patients with 300 mg oral prednisolone once-monthly for 3-6 months. [84] Pasricha has reported remarkable hair growth in one patient, refractory to other therapies with oral mini pulse, betamethasone 5 mg given as a single oral dose after breakfast on two consecutive days every week for 6 months. [85] In another study by Khaitan et al., 75% of extensive AA patients showed acceptable hair growth with betamethasone oral mini pulse. [86] Sharma et al. have used oral mini-pulse with dexamethasone with complete hair growth in 26.6% of patients and a good response in 36.6% of patients. [87] Systemic steroids in general have been found useful in recent onset disease rather than chronic AA, ophiasis, and AU. [37] Contraindications and side effects should be discussed with the patients, and the clinician should be observant to identify those at the appropriate time.
Minoxidil
Minoxidil is successful in hair regrowth by stimulating proliferation at the base of the bulb and differentiation above the dermal papilla, independent of its vascular influences. Few studies, but not all, reported successful hair growth in AA. Minoxidil is used twice-daily application, and 5% solution is more effective than 2%. [66] Young patients respond better than older patients. It was used in combination with topical or intralesional steroids or anthralin with better results. [67] It was effective in extensive AA (>50% scalp involvement) but not in AT/AU. It was generally well-tolerated, but unwanted facial hair was noticed in 3% women. [66] Pruritus or dermatitis are other rare adverse effects, and a foam formulation is less likely to induce these symptoms. [68]
Anthralin
Anthralin is an irritant, and it′s mechanism of action in AA is unknown. It is effective because of its immunosuppressive and anti-inflammatory properties by generating free radicals. [5] It is used as 0.5-1% cream with short contact therapy. It is applied daily for 20-30 minutes, for 2-3 weeks, gradually increasing contact time daily by 5 minutes up to 1 hour or till erythema and/or pruritus develops and maintained the same time of contact for 3-6 months. Thappa et al. used it as a primary choice in the treatment of patchy AA in children <10 years of age. [65] Anthralin was found effective in 75% of patchy AA and 25% of AT patients. [64] It may produce severe irritation, folliculitis, regional lymphadenopathy, and staining of skin, clothes, and hair.
Topical Immunotherapy
Topical immunotherapy is based on the principle of inducing allergic contact dermatitis by applying potent contact allergens to the affected skin. It appears that these contact sensitizers act through immunomodulation of the skin and its appendages. [5] Dinitrochlorobenzene (DNCB) was the first sensitizer used for the treatment of AA. It was found to have mutagenic effects and was not preferred. However, DNCB was found non-carcinogenic when fed in large doses in rats, mice, guinea pigs, and men. Mohan et al. reported acceptable terminal hair growth in 36% of their patients using DNCB and suggested a relook at DNCB therapy. [69] Diphenyl-cyclo-propenone (DPCP) and squaric acid dibutyl ester (SADBE) are other contact sensitizers used in AA. The efficacy of the both agents is almost same 50-60% with a range of 9-87%. [70],[71] DPCP is preferred over SADBE as it is cheap and more stable in acetone, which is a potent UV-absorber. DPCP is light- and heat-sensitive, and it should be stored in amber-colored bottles. [64] 2% solution is made by dissolving 20 mg in 1 ml of acetone, and further dilution can be prepared by diluting 2% solution with acetone taken in a pipette as per the concentration. [72] The patient is first sensitized with 2% DPCP on a 4 cm 2 area of scalp. It is left on the scalp for 1-2 days and then washed. The scalp should be protected from the sunlight during these 2 days. Two weeks later, 0.0001% DPCP is applied on to the same side of scalp and gradually concentration is increased every week, until a mild erythema or pruritus occur. [5] Once the appropriate concentration that produced allergic reaction is established, weekly application of the same concentration is continued and left for 48 hours without exposing to sun. Treatment should be continued on the same half of the scalp until the regrowth of hair, and the second half should be treated later. Once hair regrowth is complete and maintained for more than 3 months, treatment can be gradually tapered and discontinued over a period of 9 months. [102] If there is no response in 6 months, DPCP is less likely to be successful. Pruritus, erythema, scaling, postauricular lymphadenopathy, contact urticaria, post-inflammatory hyper- and hypo-pigmentation, erythema mutliforme, facial edema, and flulike symptoms are some of the side effects noted with topical immunotherapy. Pigmented contact dermatitis developing after sensitization indicates poor response to contact immunotherapy. [102] Patients should be fully informed about treatment, and a written consent should be taken. Contact with the allergen must be avoided by handlers, pharmacy, medical and nursing staff and those applying, the allergen should wear gloves and aprons. It is not recommended in pregnancy as there is no data on its safety in pregnancy.
Phototherapy
Recent Cochrane review revealed that there were not many randomized controlled studies about phototherapy in AA. [62] There are conflicting reports about efficacy of PUVA in AA. PUVA has been found to be effective in AA by decreasing the perifollicular inflammatory infiltrate. [5] Mohammad et al. has reported good or excellent response in 85% of their AA patients. [90] Turban PUVA and turban PUVASOL also have been found effective. [73],[74] PUVA in combination with oral steroids have been found effective in recalcitrant AT and AU. [37] Mild erythema, burning and increased risk for melanoma are some of the side effects observed with PUVA. NBUVB phototherapy has been found ineffective in AA. Bayramgürler et al. from Turkey reported in a recent study that only 20% showed excellent response in severe AA, most of whom received intramuscular triamcinolone acetonide injections also and concluded that NBUVB is not an effective treatment in AA. [101] 308-excimer laser has shown hair regrowth in 41.5% patches of AA, but poor results were observed in AT/AU. [103] Infrared therapy as monotherapy and in combination with other modalities has shown variable success. [104] Photodynamic therapy was not effective. [105]
Prostaglandin Analogues
Latanoprost and bimatoprost are prostaglandin analogues, which are used in open angle glaucoma caused hypertrichosis of eyelashes and hair on the malar area as an adverse effect. [75],[76] Because of this effect, these were tried in eyelash AA and found ineffective. Though the earlier studies failed to induce hair growth, a recent trial showed a cosmetically acceptable hair growth in 45% of the latanoprost-treated group. [76] Bimatoprost has also been beneficial, and Vila et al. showed cosmetically acceptable eyelash growth in 43.2% of AU patients. [77] Transient mild eye irritation or hyperemia may occur.
Topical Calcineurin Inhibitors
Topical calcinuerin inhibitors, tacrolimus, and pimecrolimus inhibit transcription following T-cell activation of several cytokines. They were tried in AA and were found to be ineffective. [97],[98]
Sulfasalazine
Sulfasalazine works as immunomodulator and immunosuppressant. It inhibits inflammatory cell chemotaxis and cytokine and antibody production. It has shown acceptable regrowth in 23%-25.6% of AA patients. [88] Aghaei et al., in an uncontrolled open label study, found complete hair regrowth in 27.3% and partial regrowth of hair in 40.9% of AA patients, and 32% developed one or other side effects. [89] Sulfasalazine can be given 0.5 g twice-daily for 1 month, followed by 1 g twice-daily for 1 month and 1.5 g twice-daily for at least 3 months. It may cause gastrointestinal distress, headache, fever, rash, hematological abnormalities, and hepatotoxicity.
Mesotherapy
Mesotherapy has become an advertised method of treatment for AA. It is given in the form of intra- or subcutaneous injections containing pharmaceutical compounds, homeopathic medicines, vitamins, nutrients, and enzymes. Shulaia et al. reported hair growth in 21 cases using nicotinic acid, vitamin C, pentoxifiline, and trace elements given over a period of 28 weeks without any side effects. [106] In contrast, patchy AA has been reported in 2 cases after receiving mesotherapy with mesoglycan and homeopathic agents. [107] Randomized controlled studies are needed to document the efficacy of mesotherapy.
Miscellaneous
Cyclosporine A causes an adverse effect of hypertrichosis in 80% of the patients used, by prolonging anagen phase of hair growth cycle. [91] But, its use in AA has shown conflicting results. Its use is limited because of side effects and high relapse rate. [64] Topical cyclosporine A 10% was tried and found to be ineffective. [108] Methotrexate had shown hair growth in 57% AA patients and when combined with prednisone, the efficacy was 63-64%. [92] Azathioprine was tried in an open label study by Farshi et al. at a dose of 2 mg/kg/day for 6 months and reported 52.3% mean regrowth, using SALT score. [93] Supplementation of anti-histamines like fexofenadine and ebastine have been found useful in AA, associated with atopic dermatitis. [37] Biologics like infliximab, etanercept, adalimumab, and efalizumab have been tried, and all of them were unsuccessful. [99] Some showed worsening or occurring of AA while on treatment with biologics. [100] Abatacept has been found effective to prevent AA by blocking T-cell activation in experimental mouse models and was proposed for further clinical tests in AA. [15]
Capsaicin was tried in a recent randomized non-blinded study in 50 patients with patchy AA in comparison with 0.05% clobetasol ointment for 6 weeks, and 9.5% showed cosmetically acceptable regrowth at week 12 in both groups. [94] A combination of topical 5% garlic gel and betamethasone 1% cream for 3 months showed statistically significant hair growth in more number of patients when compared to betamethasone and placebo. [109] Tincture iodine has been suggested as a non-specific irritant in patchy AA patients with less than 10 years of age. [65] Topical bexarotene 1% gel was evaluated in single-blinded half head study and found 50% or more partial regrowth in 12% of patients on the treated side and in 14% responded on both sides. [95] Fractional Er: Glass laser has shown complete hair regrowth in a single patient who was not responding to conventional therapies. [110] Topical tretinoin 0.05%, topical azelaic acid, inosiplex, topical onion juice, and intralesional candida antigen injections have been tried with some efficacy and need to be confirmed in large-scale, double-blind, placebo-controlled trials. [111] Liquid nitrogen cryotherapy, Chinese herbal medicine, carpronium chloride hydrate, cepharanthine, and mono-ammonium glycyrrhizinate have been used traditionally, especially in Japan, but there are no randomized controlled studies evaluating their efficacy. [37]
New drugs are being tried based on the GWAS against T-cell mechanisms and NK-cell activating ligands. Many drugs like anti-CD25, anti-CTLA-4, anti-IL-6, anti-IL-15, and Syk inhibitor, which are being evaluated in other autoimmune diseases which affect these mechanisms, can be the potential therapies for clinical trials in AA. [112]
Camouflage
At times, the treatments may not regrow the hair in AA/AT/AU in an attractive manner. [96] Camouflage techniques like hairpieces and hair additions may be a better option. Hairpieces could be in the form of wigs, demiwigs, toupees, cascades, and wiglets. Human hair wigs are most expensive, needs regular shampooing every 2-3 weeks and lasts only 2-3 years. Synthetic hair fibers may be a better option as they are less expensive, needs less maintenance, and lasts for 3-5 years. Hair additions are semi-permanent, lasting about 2 months. The natural or synthetic hair fibers are attached to the existing hair by braiding, sewing, bonding, or gluing. In case of eyelashes and eyebrows, use of artificial fibers or tattooing may be offered. These techniques are not completely free of hassles and may cause traction alopecia and breakage of hair from the glue and clips. [96]
Treatment of Children with AA
Therapeutic options in children are limited because of less tolerability and potential side-effects. Moderately potent corticosteroids are the first choice. Topical minoxidil and topical immunotherapy are the other options. Thappa et al. preferred tincture iodine and anthralin as first line. [65] Long-term use of systemic steroids is generally not recommended because of potential side effects. Psychological effects are of major concern in children, and they should be attended to with proper counseling.
Conclusion
AA is the common form of hair loss affecting the quality of life of many patients. Genetic susceptibility, environmental factors, and autoimmunity are the main etiological factors. GWAS studies had identified the key genes paving the way for better understanding of pathogenesis of AA. There is paucity of controlled studies regarding effective treatments of AA. Corticosteroids are the main stay in the treatment of AA. The other treatments are minoxidil, immunotherapy, and PUVA. Newer therapies are focused at T-cell mechanisms and NK-cell activating ligands.
| 1. |
McMichael AJ, Pearce DJ, Wasserman D, Camacho FT, Fleischer Jr AB, Feldman SR, et al. Alopecia in the United States: Outpatient utilization and common prescribing patterns. J Am Acad Dermatol 2007;57:S49-51.
[Google Scholar]
|
| 2. |
Sharma VK, Dawn G, Kumar B. Profile of alopecia areata in Northern India. Int J Dermatol 1996;35:22-7.
[Google Scholar]
|
| 3. |
Safavi KH, Muller SA, Suman VJ, Moshell AN, Melton LJ 3 rd. Incidence of alopecia areata in Olmsted County, Minnesota, 1975 through 1989. Mayo Clin Proc 1995;70:628-33.
[Google Scholar]
|
| 4. |
Tan E, Tay YK, Goh CL, Chin Giam Y. The pattern of alopecia areata in Singapore - A study of 219 Asians. Int J Dermatol 2002;41:748-53.
[Google Scholar]
|
| 5. |
Wasserman D, Guzman-Sanchez DA, Scott K, McMichael A. Alopecia areata. Int J Dermatol 2007;46:121-31.
[Google Scholar]
|
| 6. |
Al-Mutairi N, Eldin ON. Clinical profile and impact on quality of life: Seven years experience with patients of alopecia areata. Indian J Dermatol Venereol Leprol 2011;77:489-93.
[Google Scholar]
|
| 7. |
Bhat YJ, Manzoor S, Khan AR, Qayoom S. Trace element levels in alopecia areata. Indian J Dermatol Venereol Leprol 2009;75:29-31.
[Google Scholar]
|
| 8. |
Muller SA, Winkelmann RK. Alopecia areata - An evaluation of 736 patients. Arch Dermatol 1963;88:290-7.
[Google Scholar]
|
| 9. |
Alkhalifah A, Alsantali A, Wang E, McElwee KJ, Shapiro J. Alopecia areata update: Part I. Clinical picture, histopathology,and pathogenesis. J Am Acad Dermatol 2010;62:177-88.
[Google Scholar]
|
| 10. |
Tosti A, La Placa M, Placucci F, Gentilomi G, Venturoli S, Zerbini M, et al. No correlation between CMV and alopecia areata. J Invest Dermatol 1996;107:443.
[Google Scholar]
|
| 11. |
Rodriguez TA, Fernandes KE, Dresser KL, Duvic M. Concordance rate of alopecia areata in identical twins supports both genetic and environmental factors. J Am Acad Dermatol 2010;62:525-7.
[Google Scholar]
|
| 12. |
Martinez-Mir A, Zlotogorski A, Gordon D, Petukhova L, Mo J, Gilliam TC, et al. Genomewide scan for linkage reveals evidence of several susceptibility loci for alopecia areata. Am J Hum Genet 2007;80:316-28.
[Google Scholar]
|
| 13. |
Petukhova L, Duvic M, Hordinsky M, Norris D, Price V, Shimomura Y, et al. Genome-wide association study in alopecia areata implicates both innate and adaptive immunity. Nature 2010;466:113-7.
[Google Scholar]
|
| 14. |
Barahmani N, Andrade M, Slusser JP, Wei Q, Hordinsky M, Price VH, et al. Human leukocyte antigen class II alleles are associated with risk of alopecia areata. J Invest Dermatol 2008;128:240-3.
[Google Scholar]
|
| 15. |
Petukhova L, Cabral RM, Mackay-Wiggan J, Clynes R, Christiano AM. The genetics of alopecia areata: What's new and how will it help our patients? Dermatol Ther 2011;24:326-36.
[Google Scholar]
|
| 16. |
Wang E, McElwee KJ. Etiopathogenesis of alopecia areata: Why do our patients get it? Dermatol Ther 2011;24:337-47.
[Google Scholar]
|
| 17. |
Sundberg JP, Silva KA, Zhang W, Sundberg, BA, Edwards K, King LE, et al. Recombinant human hepatitis B vaccine initiating alopecia areata: Testing the hypothesis using the C3H/HeJ mouse model. Vet Dermatol 2009;20:99-104.
[Google Scholar]
|
| 18. |
Gupta MA, Gupta AK, Watteel GN. Stress and alopecia areata: A psychodermatologic study. Acta Derm Venereol 1997:77:296-8.
[Google Scholar]
|
| 19. |
Trost LB, Bergfeld WF, Calogeras E. The diagnosis and treatment of iron deficiency and its potential relationship to hair loss. J Am Acad Dermatol 2006;54:824-44.
[Google Scholar]
|
| 20. |
McElwee KJ, Sinclair R. Hair physiology and its disorders. Drug Discov Today Dis Mech 2008;5:e163-71.
[Google Scholar]
|
| 21. |
Offidani A, Amerio P, Bernardini ML, Feliciani C, Bossi G. Role of cytomegalovirus replication in alopecia areata pathogenesis. J Cutan Med Surg 2000;4:63-5.
[Google Scholar]
|
| 22. |
Bruske K, Salfeld K. Zinc and its status in some dermatological diseases: A statistical assessment. Z Hautkr 1987;62:125-31.
[Google Scholar]
|
| 23. |
Roselino AM, Almeida AM, Hippolito MA, Cerqueira BC, Maffei CM, Mfnezes JB, et al. Clinical-epidemiologic study of alopecia areata. Int J Dermatol 1996;35:181-4.
[Google Scholar]
|
| 24. |
Barahmani N, Schabath MB, Duvic M. History of atopy or autoimmunity increases risk of alopecia areata. J Am Acad Dermatol 2009;61:581-91.
[Google Scholar]
|
| 25. |
Xiao FL, Yang S, Liu JB, He PP, Yang J, Cui Y, et al. The epidemiology of childhood alopecia areata in China: A study of 226 patients. Pediatr Dermatol 2006;23:13-8.
[Google Scholar]
|
| 26. |
Leung MC, Sutton CW, Fenton DA, Tobin DJ. Trichohyalin a potential major autoantigen in human alopeciaareata. J Proteome Res 2010;9:5153-63.
[Google Scholar]
|
| 27. |
Kang H, Wu WY, Lo BK, Yu M, Leung G, Shapiro J, et al. Hair follicles from alopecia areata patients exhibit alterations in immune privilege associated gene expression in advance of hair loss. J Invest Dermatol 2010;130:2677-80.
[Google Scholar]
|
| 28. |
Gilhar A. Collapse of immune privilege in alopecia areata: Coincidental or substantial? J Invest Dermatol 2010;130:2535-7.
[Google Scholar]
|
| 29. |
Madani S, Shapiro J. Alopecia areata update. J Am Acad Dermatol 2000;42:549-66.
[Google Scholar]
|
| 30. |
Finner AM. Alopecia areata: Clinical presentation, diagnosis, and unusual cases. Dermatol Ther 2011;24:348-54.
[Google Scholar]
|
| 31. |
Sato-Kawamura M, Aiba S, Tagami H. Acute diffuse and total alopecia of the female scalp. A new subtype of diffuse alopecia areata that has a favorable prognosis. Dermatology 2002;205:367-73.
[Google Scholar]
|
| 32. |
Lew BL, Shin MK, Sim WY. Acute diffuse and total alopecia: A new subtype of alopecia areata with a favorable prognosis. J Am Acad Dermatol 2009;60:85-93.
[Google Scholar]
|
| 33. |
Yesudian P, Thambiah AS. Perinevoid alopecia. An unusual variety of alopecia areata. Arch Dermatol 1976;112:1432-4.
[Google Scholar]
|
| 34. |
Ikeda T. A new classification of alopecia areata. Dermatologica 1965;131:421-45.
[Google Scholar]
|
| 35. |
Shuster S. 'Coudability': A new physical sign of alopecia areata. Br J Dermatol 1984;111:629.
[Google Scholar]
|
| 36. |
Messenger AG. Alopecia areata. Disorders of hair. Rook's text book of dermatology. 8 th ed. In: Burns DA, Breathnach SM, Cox NH, Griffiths CE, editors. Oxford,UK: Blackwell publishing limited; 2010. p. 66.31-8.
th ed. In: Burns DA, Breathnach SM, Cox NH, Griffiths CE, editors. Oxford,UK: Blackwell publishing limited; 2010. p. 66.31-8.'>[Google Scholar]
|
| 37. |
Ito T. Advances in the management of alopecia areata. J Dermatol 2012;39:11-7.
[Google Scholar]
|
| 38. |
Tosti A, Whiting D, Iorizzo M, Pazzaglia M, Misciali C, Vincenzi C, et al. The role of scalp dermoscopy in the diagnosis of alopecia areata incognita. J Am Acad Dermatol 2008;59:64-7.
[Google Scholar]
|
| 39. |
Inui S, Nakajima T, Nakagawa K, Itami S. Clinical significance of dermoscopy in alopecia areata: Analysis of 300 cases. Int J Dermatol 2008;47:688-93.
[Google Scholar]
|
| 40. |
Mane M, Nath AK, Thappa DM. Utility of dermoscopy in alopecia areata. Indian J Dermatol 2011;56:407-11.
[Google Scholar]
|
| 41. |
Inui S, Nakajima T, Itami S. Coudability hairs: A revisited sign of alopecia areata assessed by trichoscopy. Clin Exp Dermatol 2010;35:361-5.
[Google Scholar]
|
| 42. |
SharmaVK, Dawn G, Muralidhar S, Kumar B. Nail changesin 1000 Indian patients with alopecia areata. J Eur Acad Dermatol Venereol 1998;10:189-91.
[Google Scholar]
|
| 43. |
Gandhi V, Baruah MC, Bhattacharaya SN. Nail changes in alopecia areata: Incidence and pattern. Indian J Dermatol Venereol Leprol 2003;69:114-5.
[Google Scholar]
|
| 44. |
Kasumagic-Halilovic E, Prohic A. Nail changes in alopecia areata: Frequency and clinical presentation. J Eur Acad Dermatol Venereol 2009;23:240-1.
[Google Scholar]
|
| 45. |
Dotz WI, Leiber CD, Wogt PJ. Leuconychia punctata and pitted nails in alopecia areata. Arch Dermatol 1985;121:1452-4.
[Google Scholar]
|
| 46. |
Hordinsky M, Ericson M. Autoimmunity: Alopecia areata. J Investig Dermatol Symp Proc 2004;9:73-8.
[Google Scholar]
|
| 47. |
Kasumagic-Halilovic E. Thyroid autoimmunity in patientswith alopecia areata. Acta Dermatovenerol Croat 2008;16:123-5.
[Google Scholar]
|
| 48. |
Thomas EA, Kadyan RS. Alopecia areata and autoimmunity: A clinical study. Indian J Dermatol 2008;53:70-4.
[Google Scholar]
|
| 49. |
Goh C, Finkel M, Christos PJ, Sinha AA. Profile of 513 patients with alopecia areata: Associations of disease subtypes with atopy, autoimmune disease and positive family history. J Eur Acad Dermatol Venereol 2006;20:1055-60.
[Google Scholar]
|
| 50. |
Wang SJ, Shohat T, Vadheim C, Shellow W, Edwards J, Rotter JI. Increased risk for type I (insulin-dependent) diabetes in relatives of patients with alopecia areata (AA). Am J Med Genet 1994;51:234-9.
[Google Scholar]
|
| 51. |
Chu SY, Chen YJ, Tseng WC, Lin MW, Chen TJ, Hwang CY, et al. Comorbidity profiles among patients with alopecia areata: The importance of onset age, a nationwide population-based study. J Am Acad Dermatol 2011;65:949-56.
[Google Scholar]
|
| 52. |
Dubois M, Baumstarck-Barrau K, Gaudy-Marqueste C, Richard MA, Loundou A, Auquier P, et al. Quality of life in alopecia areata: A study of 60 Cases. J Invest Dermatol 2010;130:2830-3.
[Google Scholar]
|
| 53. |
Pandhi D, Singal A, Gupta R, Das G. Ocular alterations in patients of alopecia areata. J Dermatol 2009;36:262-8.
[Google Scholar]
|
| 54. |
Dy LC, Whiting DA. Histopathology of alopecia areata, acute and chronic: Why is it important to the clinician?t Dermatol Thera 2011;24:369-7.
[Google Scholar]
|
| 55. |
Chaitra V, Rajalakshmi T, Kavdia R. Histopathologic profile of alopecia areata in Indian patients. Int J Trichol 2010;2:14-7.
[Google Scholar]
|
| 56. |
Whiting DA. Structural abnormalities of the hair shaft. J Am Acad Dermatol 1987;16:1-25.
[Google Scholar]
|
| 57. |
Gupta LK, Khare AK, Garg A, Mittal A. Congenital triangular alopecia: A close mimicker of alopecia areata. Int J Trichol 2011;3:40-1.
[Google Scholar]
|
| 58. |
Olsen EA. Investigative guidelines for alopecia areata. Dermatol Ther 2011;24:311-9.
[Google Scholar]
|
| 59. |
Bartels NG, Jahnke I, Patzelt A, Richter H, Lademann J, Blume-Peytavi U. Hair shaft abnormalities in alopecia areata evaluated by optical coherence tomography. Skin Res Technol 2011;17:201-5.
[Google Scholar]
|
| 60. |
Olsen EA, Hordinsky MK, Price VH, Roberts JL, Shapiro J, Canfield D, et al. National Alopecia Areata Foundation. Alopecia areata investigational assessment guidelines. Part II. National Alopecia Areata Foundatin. J Am Acad Dermatol 2004;51:440-7.
[Google Scholar]
|
| 61. |
Tosti A, Bellavista S, Iorizzo M. Alopecia areata: A long term follow-up study of 191 patients. J Am Acad Dermatol 2006;55:438-41.
[Google Scholar]
|
| 62. |
Delamere FM, Sladden MJ, Dobbins HM, Leonardi-Bee J. Interventions for alopecia areata (Review). Cochrane Database Syst Rev 2008;CD004413.
[Google Scholar]
|
| 63. |
Tosti A, Piraccini BM, Pazzaglia M, Vincenzi C. Clobetasol propionate 0.05% under occlusion in the treatment of alopecia totalis/universalis. J Am Acad Dermatol 2003;49:96-8.
[Google Scholar]
|
| 64. |
AlKhalifah A, Alsantali A, Wang E, McElwee KJ, Shapiro J. Alopecia areata update: Part II. Treatment. J Am Acad Dermatol 2010;62:191-202.
[Google Scholar]
|
| 65. |
Thappa DM, Vijayikumar M. Alopecia areata. Indian J Dermatol Venereol Leprol 2001;67:188.
[Google Scholar]
|
| 66. |
Lucky AW, Piacquadio DJ, Ditre CM, Dunlap F, Kantor I, Pandya AB, et al. A randomized, placebo-controlled trial of 5% and 2% topical minoxidil solutions in the treatment of female pattern hair loss. J Am Acad Dermatol 2004;50:541-53.
[Google Scholar]
|
| 67. |
Fiedler VC, Wendrow A, Szpunar GJ, Metzler C, DeVillez RL. Treatment resistant alopecia areata. Response to combination therapy with minoxidil plus anthralin. Arch Dermatol 1990;126:756-9.
[Google Scholar]
|
| 68. |
Olsen EA, Whiting D, Bergfeld W, Miller J, Hordinsky M, Wanser R, et al. A multicenter, randomized, placebo-controlled, double-blind clinical trial of a novel formulation of 5% minoxidil topical foam versus placebo in the treatment of androgenetic alopecia in men. J Am Acad Dermatol 2007;57:767-74.
[Google Scholar]
|
| 69. |
Mohan KH, Balachandran C, Shenoi SD, Rao R, Sripathi H, Prabhu S. Topical dinitrochlorobenzene (DNCB) for alopecia areata: Revisited. Indian J Dermatol Venereol Leprol 2008;74:401-2.
[Google Scholar]
|
| 70. |
Avgerinou G, Gregoriou S, Rigopoulos D, Stratigos A, Kalogeromitros D, Katsambas A. Alopecia Areata: Topical immunotherapy treatment with diphencyprone. J Eur Acad Dermatol Venereol 2008;22:320-3.
[Google Scholar]
|
| 71. |
Singh G, Lavanya MS. Topical immunotherapy in alopecia areata. Int J Trichol 2010;2:36-9.
[Google Scholar]
|
| 72. |
Muthuvel K. Practicality in using diphenyl cyclo propenone for alopecia areata. Int J Trichol 2011;3:96-7.
[Google Scholar]
|
| 73. |
Behrens-Williams SC, Leiter U, Schiener R, Weidmann M, Peter RU, Kerscher M. The PUVA-turban as a new option of applying a dilute psoralen solution selectively to the scalp of patients with alopecia areata. J Am Acad Dermatol 2001;44:248-52.
[Google Scholar]
|
| 74. |
Sornakumar L, Sekar CS, Srinivas CR. Turban PUVASOL: An effective treatment in alopecia totalis. Int J Trichol 2010;2:106-7.
[Google Scholar]
|
| 75. |
Tosti A, Pazzaglia M, Voudouris S, Tosti G. Hypertrichosis of the eyelashes caused by bimatoprost. J Am Acad Dermatol 2004;51:S149-50.
[Google Scholar]
|
| 76. |
Coronel-Perez IM, Rodriguez-Rey EM, Camacho-Martinez FM. Latanoprost in the treatment of eyelash alopecia in alopecia areata universalis. J Eur Acad Dermatol Venereol 2010;24:481-5.
[Google Scholar]
|
| 77. |
Vila TO, Camacho Martinez FM. Bimatoprost in the treatment of eyelash universalis alopecia areata. Int J Trichol 2010;2:86-8.
[Google Scholar]
|
| 78. |
Shapiro J, Price VH. Hair regrowth. Therapeutic agents. Dermatol Clin 1998;16:341-56.
[Google Scholar]
|
| 79. |
Sohn KC, Jang S, Choi DK, Lee YS, Yoon TJ, Jeon EK, et al. Effect of thioredoxin reductase 1 on glucocorticoid receptor activity in human outer root sheath cells. Biochem Biophys Res Commun 2007;356:810-5.
[Google Scholar]
|
| 80. |
Friedli A, Labarthe MP, Engelhardt E, Feldmann R, Salomon D, Saurat JH. Pulse methylprednisolone therapy for severe alopecia areata: An open prospective study of 45 patients. J Am Acad Dermatol 1998;39:597-602.
[Google Scholar]
|
| 81. |
Kar BR, Handa S, Dogra S, Kumar B. Placebo-controlled oral pulse prednisolone therapy in alopecia areata. J Am Acad Dermatol 2005;52:287-90.
[Google Scholar]
|
| 82. |
Kurosawa M, Nakagawa S, Mizuashi M, Sasaki Y, Kawa- mura M, Saito M, et al. A comparison of the efficacy, relapse rate and side effects among three modalities of systemic corticosteroid therapy for alopecia areata. Dermatology 2006;212:361-5.
[Google Scholar]
|
| 83. |
Thappa DM, Vijaikumar S. Intravenous dexamethasone pulse therapy for extensive alopecia areata. Indian J Dermatol 1999;44:187-90.
[Google Scholar]
|
| 84. |
Sharma VK. Pulsed administration of corticosteroids in the treatment of alopecia areata. Int J Dermatol 1996;35:133-6.
[Google Scholar]
|
| 85. |
Pasricha JS, Kumrah L. Alopecia totalis treated with oral mini-pulse (OMP) therapy with betamethasone. Indian J Dermatol Venereol Leprol 1996;62:106-9.
[Google Scholar]
|
| 86. |
Khaitan BK, Mittal R, Verma KK. Extensive alopecia areata treated with betamethasone oral mini-pulse therapy: An open uncontrolled study. Indian J Dermatol Venereol Leprol 2004;70:350-3.
[Google Scholar]
|
| 87. |
Sharma VK, Gupta S. Twice weekly dexamethasone oral pulse in the treatment of extensive alopecia areata. J Dermatol 1999;26:562-5.
[Google Scholar]
|
| 88. |
Rashidi T, Mahd AA. Treatment of persistent alopecia areata with sulfasalazine. Int J Dermatol 2008;47:850-2.
[Google Scholar]
|
| 89. |
Aghaei S. An uncontrolled, open label study of sulfasalazine in severe alopecia areata. Indian J Dermatol Venereol Leprol 2008;74:611-3.
[Google Scholar]
|
| 90. |
Mohamed Z, Bhouri A, Jallouli A, Fazaa B, Kamoun MR, Mokhtar I, et al. Alopecia areata treatment with a phototoxic dose of UVA and topical 8-methoxypsoralen. J Eur Acad Dermatol Venereol 2005;19:552-5.
[Google Scholar]
|
| 91. |
Ferrando J, Grimalt R. Partial response of severe alopecia areata to cyclosporine A. Dermatology 1999;199:67-9.
[Google Scholar]
|
| 92. |
Chartaux E, Joly P. Long-term follow-up of the efficacy of methotrexate alone or in combination with low doses of oral corticosteroids in the treatment of alopecia areata totalis or universalis. (Article in French). Ann Dermatol Venereol 2010;137:507-13.
[Google Scholar]
|
| 93. |
Farshi S, Mansouri P, Safar F, Khiabanloo SR. Could azathioprine be considered as a therapeutic alternative in the treatment of alopecia areata? A pilot study. Int J Dermatol 2010;49:1188-93.
[Google Scholar]
|
| 94. |
Ehsani A, Toosi S, Seirafi H, Akhyani M, Hosseini M, Azadi R, et al. Capsaicin vs. Clobetasol for the treatment of localized alopecia areata. J Eur Acad Dermatol Venereol 2009;23:1451-3.
[Google Scholar]
|
| 95. |
Talpur R, Vu J, Bassett R, Stevens V, Duvic M. Phase I/II randomized bilateral half-head comparison of topical bexarotene 1% gel for alopecia areata. J Am Acad Dermatol 2009;61:592.e1-9.
[Google Scholar]
|
| 96. |
Draelos ZD. Camouflage technique for alopecia areata: What is a patient to do? Dermatol Ther 2011;24:305-10
[Google Scholar]
|
| 97. |
Price VH,Willey A, Chen BK. Topical tacrolimus in alopecia areata. J Am Acad Dermatol 2005;52:138-9.
[Google Scholar]
|
| 98. |
Rigopoulos D, Gregoriou S, Korfitis C, Gintzou C, Vergou T, Katrinaki A, et al. Lack of response of alopecia areata to pimecrolimus cream. Clin Exp Dermatol 2007;32:456-7.
[Google Scholar]
|
| 99. |
Otberg N. Systemic treatment for alopecia areata. Dermatol Ther 2011;24:320-5.
[Google Scholar]
|
| 100. |
Kirshen C, Kanigsberg N. Alopecia areata following adalimumab. J Cutan Med Surg 2009;13:48-50.
[Google Scholar]
|
| 101. |
Bayramgürler D, Demirsoy EO, Aktürk AS, Kýran R. Narrowband ultraviolet B phototherapy for alopecia areata. Photodermatol Photoimmunol Photomed 2011;27:325-7.
[Google Scholar]
|
| 102. |
Inui S, Nakajima T, Toda N, Itami S. Pigmented contact dermatitis due to therapeutic sensitizer as complication of contact immunotherapy in alopecia areata. J Dermatol 2010;37:888-93.
[Google Scholar]
|
| 103. |
Al-Mutairi N. 308-nm excimer laser for the treatment of alopecia areata. Dermatol Surg 2007;33:1483-7.
[Google Scholar]
|
| 104. |
Tzung TY, Chen CY, Tzung TY, Kao FJ, Chen WC. Infrared irradiation as an adjuvant therapy in recalcitrant alopecia areata. Dermatol Surg 2009;35:721-3.
[Google Scholar]
|
| 105. |
Fernandez-Guarino M, Harto A, Garcia-Morales I, Perez-Garcia B, Arrazola JM, Jaen P. Failure to treat alopecia areata with photodynamic therapy. Clin Exp Dermatol 2008;33:585-7.
[Google Scholar]
|
| 106. |
Shulaia T, Kiladze N, Babilashvili I.Mesotherapy in treatment of alopecia areata. J Exp Clin Med 2007;1:19-22.
[Google Scholar]
|
| 107. |
Duque-Estrada B, Vincenzi C, Misciali C, Tosti A. Alopecia secondary to mesotherapy. J Am Acad Dermatol 2009;61:707-9.
[Google Scholar]
|
| 108. |
Gilhar A, Pillar T, Etzioni A. Topical cyclosporin A in alopecia areata. Acta DermVenereol 1989;69:252-3.
[Google Scholar]
|
| 109. |
Hajheydari Z, Jamshidi M, Akbari J, Mohammadpour R. Combination of topical garlic gel and betamethasone valerate cream in the treatment of localized alopecia areata: A double-blind randomized controlled study. Indian J Dermatol Venereol Leprol 2007;73:29-32.
[Google Scholar]
|
| 110. |
Yoo KH, Kim MN, Kim BJ, Kim CW. Treatment of alopecia areata with fractional photothermolysis laser. Int J Dermatol 2010;49:845-7.
[Google Scholar]
|
| 111. |
Alsantali A, Alopecia areata: A new treatment plan. Clin Cosmet Investig Dermatol 2011;4:107-15.
[Google Scholar]
|
| 112. |
Hordinsky MK. Treatment of alopecia areata:"What is new on the horizon?" Dermatol Ther 2011;24:364-8.
[Google Scholar]
|
Fulltext Views
56,232
PDF downloads
10,037





