Translate this page into:
Role of polymerase chain reaction in detection of genital herpes
2 Department of Dermatology, Venereology and Leprology, Postgraduate Institute of Medical Education and Research, Chandigarh, India
Correspondence Address:
Radha Kanta Ratho
Department of Virology, Postgraduate Institute of Medical Education and Research, Chandigarh - 160 012
India
| How to cite this article: Goyal K, Ratho RK, Kanwar A J, Mishra B, Singh MP. Role of polymerase chain reaction in detection of genital herpes. Indian J Dermatol Venereol Leprol 2013;79:705-706 |
Sir,
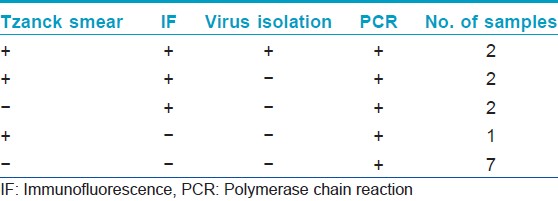
Genital herpes is a significant public health disease worldwide. Clinical diagnosis of genital herpes simplex virus (HSV) infection is insensitive and non-specific and requires laboratory confirmation. We evaluated the utility of polymerase chain reaction (PCR) for the diagnosis of genital herpes in 25 patients suspected of genital herpes. After taking an informed consent, swab samples were collected from the genital ulcers for Tzanck smear, antigen detection by indirect immunofluorescence (indirect IF), virus isolation in vero cell line and HSV DNA detection by targeting Glycoprotein D region by nested PCR as described previously by Read and Kurtz. [1] The blood samples were collected using aseptic conditions for antibody detection (IgG and IgM) by Enzyme-linked immunosorbent assay (ELISA). All the enrolled patients were prescribed acyclovir by the dermatologist. Positive control (tissue culture isolates of HSV-1 and HSV-2) and negative control (reaction mix containing no DNA) were included in each run to rule out false negative and false positive reactions. Among the 25 subjects, male:female ratio was 11.5:1 with a mean age of 32.8 years. Three patients were found to be human immunodeficiency virus positive on routine screening. None of the patients were reactive to Venereal Disease Research Laboratory test (VDRL). Majority (92%) of the patients presented with recurrent infection. Minute painful genital ulcers were the common presentation in each patient with multiple lesions in 80% of them. Five patients (20%) showed the presence of multinucleated giant cells in Tzanck smear examination. HSV-2 antigen was demonstrated by indirect IF in 6 (24%) patients. None of the patients showed the presence of HSV-1 antigen. The cytopathological effect (CPE) suggestive of HSV was observed on second passage in 2 (8%) samples. The CPE was confirmed by indirect IF, both the samples were positive for HSV-2. All the patients demonstrated IgG antibodies but none of the patients showed IgM specific antibodies, against HSV type 1 or 2. Following nested DNA PCR, the 272 bp amplified product was visualized in ethidium bromide stained gel showing the presence of HSV DNA in 14 (56%) samples [Figure - 1]. The sensitivity of conventional methods, i.e., Tzanck smear, type specific antigen detection and virus isolation was 35.71%, 42.85% and 14.28% respectively when compared to HSV DNA detection by PCR. The DNA detection by PCR was able to detect an additional 12 (48%) cases, which were missed by tissue culture alone and 8 (32%) cases, which were missed by indirect-IF, 9 (36%) cases, which were missed by Tzanck smear alone. Thus, overall PCR was able to detect 7 (28%) additional cases which were missed by all the conventional techniques [Table - 1].
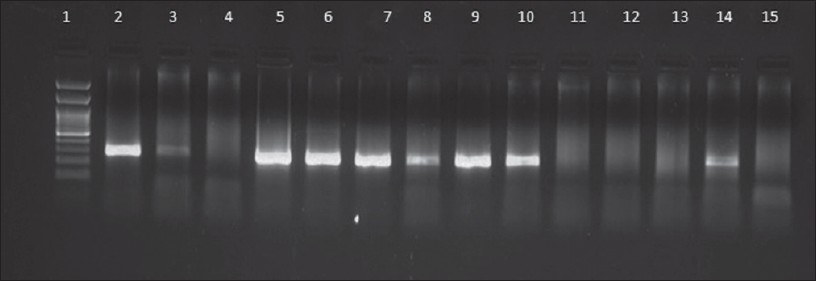 |
| Figure 1: Results of HSV PCR in representative genital herpes patients. Lane 1: Molecular marker (100 bp), Lane 2: Positive control (272 bp), Lane 3, 5, 6, 7, 8, 9, 10, 14: Positive samples, Lane 4, 11, 12, 13: Negative samples, Lane 15: Negative control |
Though, virus isolation in cell culture has been considered the gold standard for diagnosis of early genital infections but has several limitations. It is less reliable in patients presenting with ulceration, crusting or reactivations. [2] In the present study prior acyclovir treatment was reported in 57.1% patients (8/14) and intermittent shedding of virus might have been missed as samples were collected at one point of time. Though all precautions were taken to maintain the cold chain during transportation of samples, but it is well known that positivity is higher during cooler weather. [3] However, nucleic acid tests are much less affected by specimen storage beyond 48 h, freezing, thawing or bacterial contamination. Presently, the patients had HSV specific IgG antibodies but none had IgM antibodies. This may be due to the effect of prior antiviral treatment before presented to sexually transmitted diseases (STD) clinic (11/25) and it is well known that specific antivirals might inhibit the production of HSV specific type 2 antibodies. [4]
Immunofluorescence is known to be a better alternate and can give results within 6-8 h but it requires expertise to visualize the slides. Published studies have shown PCR to be 4.1 times more sensitive than culture in detecting HSV infection. [3] PCR has been reported with increased sensitivity by 13.3% in specimens from vesicular lesions, by 27.4% from ulcerative lesions and by 20% from crusting lesions. [5] Though PCR has been well characterized as a method for rapid and sensitive diagnosis of HSV. However, its role has been largely confined to the investigation of suspected HSV encephalitis where it has replaced virus culture as the gold standard. The additional advantage of PCR in case of genital herpes would be the detection of the virus in subclinical episodes of virus shedding and in undiagnosed symptomatic genital lesions. The major limitation of the present study is small sample size and large prospective studies are required to replace virus culture by PCR as the gold standard in diagnosis of genital herpes.
| 1. |
Read SJ, Kurtz JB. Laboratory diagnosis of common viral infections of the central nervous system by using a single multiplex PCR screening assay. J Clin Microbiol 1999;37:1352-5.
[Google Scholar]
|
| 2. |
Corey L. The current trend in genital herpes. Progress in prevention. Sex Transm Dis 1994;21:S38-44.
[Google Scholar]
|
| 3. |
Wald A, Huang ML, Carrell D, Selke S, Corey L. Polymerase chain reaction for detection of herpes simplex virus (HSV) DNA on mucosal surfaces: Comparison with HSV isolation in cell culture. J Infect Dis 2003;188:1345-51.
[Google Scholar]
|
| 4. |
Bryson YJ. Current status and prospects for oral acyclovir treatment of first episode and recurrent genital herpes simplex virus. J Antimicrob Chemother 1983;12 Suppl B: 61-5.
[Google Scholar]
|
| 5. |
Scoular A. Using the evidence base on genital herpes: Optimising the use of diagnostic tests and information provision. Sex Transm Infect 2002;78:160-5.
[Google Scholar]
|
Fulltext Views
2,278
PDF downloads
1,592





