Translate this page into:
A case of late onset dermal melanocytosis in the C3 dermatome
2 Department of Pathology, School of Medicine, Akdeniz University, Antalya, Turkey
3 Department of Neurology, School of Medicine, Akdeniz University, Antalya, Turkey
Correspondence Address:
Ayse Akman-Karakas
Department of Dermatology and Venereology, School of Medicine, Akdeniz University, 07058, Antalya
Turkey
| How to cite this article: �zkesici B, �nal B, Akman-Karakas A, Bassorgun CI, Uysal H, Alpsoy E. A case of late onset dermal melanocytosis in the C3 dermatome. Indian J Dermatol Venereol Leprol 2016;82:705-707 |
Sir,
A 55-year-old woman reported to our clinic for asymptomatic bluish pigmentation over the left ear lobe which first appeared 6 years ago and gradually extended to involve the helix, the postauricular area and neck. There was no history of preceding inflammation, trauma or cosmetic use except for hair dye. She denied a family history of similar lesions. On dermatologic examination, there was bluish-gray, mottled macular pigmentation affecting the whole of the left helix, left ear lobe, left postauricular area and neck corresponding to the dermatome innervated by the third cervical spinal nerve [Figure - 1]a and [Figure - 1]b. There was no mucosal involvement. Neurologic examination revealed lesional hypoesthesia when compared with the trigeminal areas of the face, contralateral ear lobe and post-auricular skin.
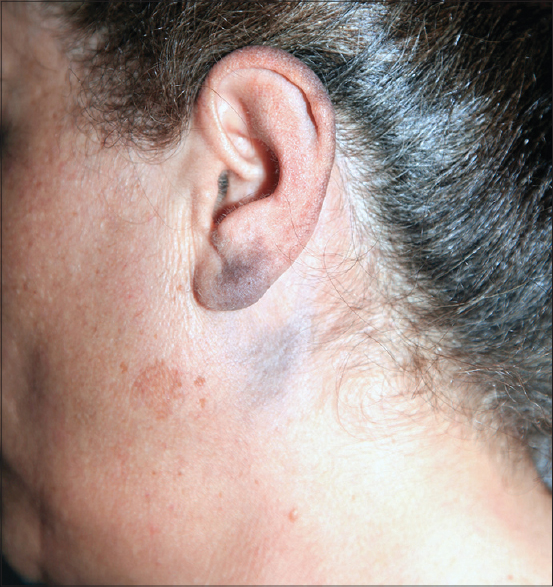 |
| Figure 1a: Blue-gray, mottled macular pigmentation affecting the left helix |
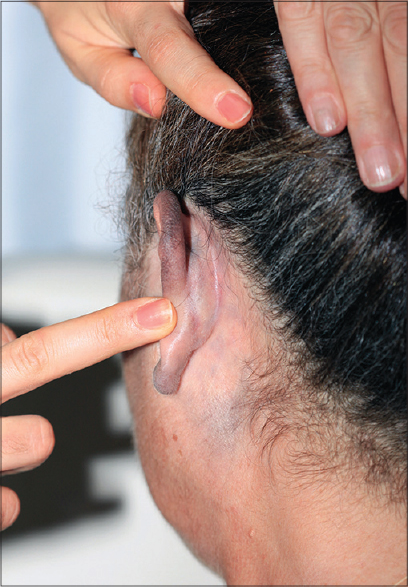 |
| Figure 1b: Left ear lobe, left postauricular area and neck |
Urinary homogentisic acid level was analyzed to rule out ochronosis and was within the normal range (0.37 mmol/mol creatinine). The complete blood count and routine biochemical tests were also normal. A skin biopsy from the lesional skin on the ear lobe showed numerous dendritic melanocytes scattered throughout the dermis without an increase in the number of melanophages [Figure - 2]a. Immunohistochemical examination showed that the dermal dendritic cells were immunoreactive for Melan A [Figure - 2]b with a low Ki-67 proliferation index, interpreted as dermal melanocytosis.
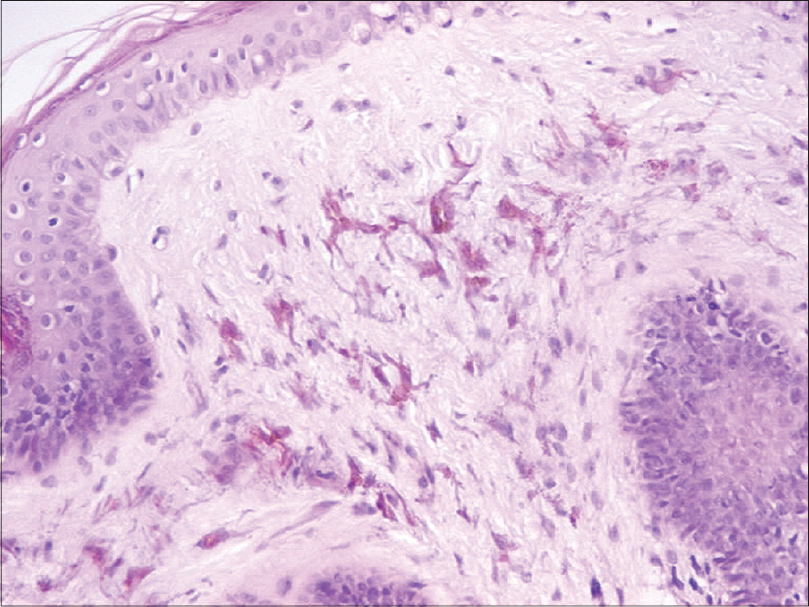 |
| Figure 2a: Dendritic, heavily pigmented melanocytes (H and E, ×200) |
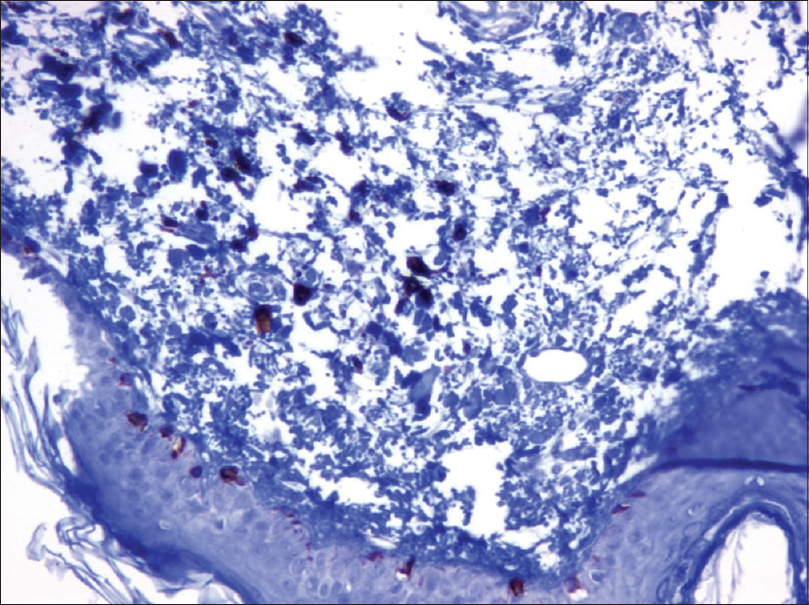 |
| Figure 2b: Dendritic melanocytes in the upper dermis show Melan-A positivity (IHC, ×200) |
Dermal melanocytosis is a condition characterized by intradermal dendritic melanocytes and may be either congenital or acquired. Most cases including nevus of Ota, nevus of Ito and Mongolian spots present at birth or in early childhood. Acquired dermal melanocytosis is a rare condition which appears in adult life and consists of the nevus of Hori and Takayama, the nevus of Sun and an extrafacial acquired dermal melanocytosis. The dermal melanocytoses are characterized by the classical speckled or mottled, gray or blue-grey pigmentation which is caused by the Tyndall effect. Diagnosis is based on clinical features since all have similar histopathological findings, i.e. dendritic dermal melanocytes. The only exception is the blue nevus which also contains a variable number of melanophages.[1]
The nevus of Ota, also known as oculodermal melanocytosis and nevus fusco-caeruleus ophthalmo-maxillaris, involves the dermatome innervated by the first (ophthalmic) and second (maxillary) divisions of the trigeminal nerve. The conjunctiva, sclera, tympanic membrane, or oral and nasal mucosa of the affected dermatomes may be involved in the nevus of Ota which usually presents at birth or appears around puberty.[1] Some cases of nevus of Ota with adult onset have also been reported.[2] A very rare case of ipsilateral sensorineural hearing loss related to neurologic involvement has been reported.[3] The nevus of Ito, also known as the fusco-caeruleus acromio-deltoideus, involves the skin innervated by the posterior supraclavicular and lateral brachial nerves. It is otherwise clinically and histopathologically similar to the nevus of Ota.[4],[5] Adult onset cases have also been reported rarely in this condition.[6] In other words, the late onset form of these two congenital dermal melanocytosis has only been reported very rarely.
We could find only two cases of extrafacial dermal melanocytosis with C3 dermatomal distribution similar to our case [Table - 1].[7],[8] In the first case, a 27-year-old woman presented with a 10-year history of asymptomatic blue discoloration in a distribution exactly similar to our case. In the second case, a 2-year-old boy presented with pigmented gray-blue macules on his right auricle that appeared at birth. Neither case report contained any information about neurological examination.[3] Recent data and our experience emphasize the importance of neurological examination and could serve as evidence that unilateral dermatomal diseases might be associated with neurological involvement.[9]

The pathogenesis of the disease is poorly understood, however, three hypotheses have been proposed. The first hypothesis is that dermal melanocytes appear when melanocytes migrating from the neural crest during embryological development fail to reach their proper location in the basal layer of the epidermis.[10] Neurons of the sensory ganglion and melanocytes have the same embryonic origin, the neural crest. Therefore, arrest of migration may affect neurons of the sensory ganglion beside the melanocytes. This theory can explain hypoesthesia on the lesional skin in our case and the ipsilateral sensorineural hearing loss in a case of nevus of Ota.[3] The accumulation of melanocytes in unusual locations and their effect on normal biological behavior might play a role in the development of hypoesthesia. The second hypothesis for dermal melanocytosis is that dermal melanocytes may be dropped from the epidermis or migrate from follicular bulbs.[1] The third is the reactivation of pre-existing latent dermal melanocytes triggered by some unknown factors. The presence of melanocytes in the dermis of uninvolved skin adjacent to lesions supports this theory.[11] It suggests that dormant dermal melanocytes may be present and remain unnoticed from birth until the melanin synthesizing pathway is activated by inflammation, local trauma, sex hormones, aging or some unknown stimuli. Sun damage may also have a contributory role in the pathogenesis, considering the photoexposed distribution of lesions. A much higher incidence among Asians than other populations and positive family history in some cases may lend support to some yet unknown genetic factors in the pathogenesis.[12]
Our case report highlights that dermal melanocytosis only rarely manifests clinically in adult life and these patients should undergo a thorough neurological evaluation to rule out associated neurological disorders.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
| 1. |
Hori Y, Takayama O. Circumscribed dermal melanoses. Classification and histologic features. Dermatol Clin 1988;6:315-26.
[Google Scholar]
|
| 2. |
Lynn A, Brozena SJ, Espinoza CG, Fenske NA. Nevus of Ota acquisita of late onset. Cutis 1993;51:194-6.
[Google Scholar]
|
| 3. |
Alvarez-Cuesta CC, Raya-Aguado C, Vázquez-López F, García PB, Pérez-Oliva N. Nevus of Ota associated with ipsilateral deafness. J Am Acad Dermatol 2002;47 5 Suppl: S257-9.
[Google Scholar]
|
| 4. |
Newton Bishop JA. Lentigos, melanocytic naevi and melanoma. In: Burns T, Breathnach S, Cox N, Christopher Griffiths C. editors. Rook's Textbook of Dermatology. 8th ed., Ch. 54. Singapore: Wiley-Blackwell; 2010.
[Google Scholar]
|
| 5. |
Ito M. Nevus fusco-caeruleus acromio-deltoideus. Tohoku J Exp Med 1954;60:10.
[Google Scholar]
|
| 6. |
Mataix J, López N, Haro R, González E, Angulo J, Requena L. Late-onset Ito's nevus: An uncommon acquired dermal melanocytosis. J Cutan Pathol 2007;34:640-3.
[Google Scholar]
|
| 7. |
Abbott RA, Stefanato CM, Scarisbrick JJ. Nevoid dermal melanocytosis in the dermatome C3. J Am Acad Dermatol 2009;60:1072-3.
[Google Scholar]
|
| 8. |
Mizuashi M, Suetake T, Aiba S, Tagami H. Dermal melanocytosis of the helix. Pediatr Dermatol 2010;27:305-6.
[Google Scholar]
|
| 9. |
Akman-Karakas A, Kandemir H, Senol U, Unal A, Duman O, Ciftcioglu MA, et al. Unilateral nevoid telangiectasia accompanied by neurological disorders. J Eur Acad Dermatol Venereol 2011;25:1356-9.
[Google Scholar]
|
| 10. |
Roth B, Grezard P, Balme B, Kanitakis J, Perrot H. Acquired dermal melanocytosis: Clinical, pathological and ultrastructural study. Ann Dermatol Venereol 2002;129 (4 Pt 1):409-12.
[Google Scholar]
|
| 11. |
Mizushima J, Nogita T, Higaki Y, Horikoshi T, Kawashima M. Dormant melanocytes in the dermis: Do dermal melanocytes of acquired dermal melanocytosis exist from birth? Br J Dermatol 1998;139:349-50.
[Google Scholar]
|
| 12. |
Mizoguchi M, Murakami F, Ito M, Asano M, Baba T, Kawa Y, et al. Clinical, pathological, and etiologic aspects of acquired dermal melanocytosis. Pigment Cell Res 1997;10:176-83.
[Google Scholar]
|
Fulltext Views
5,105
PDF downloads
1,875





