Translate this page into:
A preliminary prospective non-randomized controlled trial to compare the efficacy of subcutaneous etanercept versus oral methotrexate in moderate-to-severe chronic plaque psoriasis and correlation of response with T helper (th) 1, th2, th17 and T regulatory cytokine patterns
2 Department of Biochemistry, All India Institute of Medical Sciences, New Delhi, Indi, India
Correspondence Address:
Sujay Khandpur
Department of Dermatology and Venereology, All India Institute of Medical Sciences, East Ansari Nagar, New Delhi - 110 029
India
| How to cite this article: Khandpur S, Singh A, Kumar A, Sharma A. A preliminary prospective non-randomized controlled trial to compare the efficacy of subcutaneous etanercept versus oral methotrexate in moderate-to-severe chronic plaque psoriasis and correlation of response with T helper (th) 1, th2, th17 and T regulatory cytokine patterns. Indian J Dermatol Venereol Leprol 2020;86:441-445 |
Sir,
Psoriasis involves immune dysregulation characterized by dominance of T-helper 1 (Th1) and downregulation of Th2 response.[1],[2] Some studies have reported that biological agents correct this cytokine imbalance, whereas conventional medications do not.[3] Various trials have compared etanercept with methotrexate in rheumatoid and psoriatic arthritis; however, we were unable to find any head-to-head trials in plaque psoriasis.
This was a preliminary prospective nonrandomized trial comparing etanercept with methotrexate in moderate-to-severe plaque psoriasis. Consecutive patients of moderate to severe psoriasis with PASI >7 except erythrodermic or pustular variants aged between 18 to 72 years seen in the Department of Dermatology, All India Institute of Medical Sciences, New Delhi, India, were included. Institutional ethics committee approved it (IEC/RP 46/2017), and the patients gave informed consent.
Patients in Group A received injection etanercept (Enbrel, Pfizer) [maintained in a proper cold chain], 50 mg twice weekly subcutaneously at the hospital day-care facility. The injection was provided free of cost to 20 patients until week 2, 18 patients until week 4, 13 patients until week 8, and 7 patients until week 12. The analysis is only of patients who received etanercept. In the methotrexate group of 28 patients included, 21 patients completed treatment till 8 weeks and 19 till 12 weeks. Mantoux positive (≥15 mm) patients (n = 4), received adequate prophylaxis with anti-tubercular drugs (ATD) (600 mg rifampicin and 300 mg isoniazid daily for 4 months) along with etanercept. In Group B, 28 patients received oral methotrexate 15 mg/week. Both groups received vaseline as the sole topical agent. All patients were followed up at 2, 4, 8 and 12 weeks. Treatment response was assessed using PASI, patient global assessment (PGA) and investigator global assessment (IGA). Estimation of serum cytokines (IL-4, IL-17, IFN-γ, TGF-ß and TNF-α) by sandwich ELISA (G Biosciences, USA) and tissue cytokines (IL-4 and IFN-γ) from lesional skin by real-time PCR was undertaken at baseline and week 12.
Ststistical analysis per protocol was done using Stata version 14.1 software.
Baseline profile of patients was comparable in both groups [Table - 1]. Median PASI in etanercept group reduced from 12.06 to 4 (P = 0.001) and in methotrexate group, from 12.65 to 0.9 (P < 0.001) at week 8. There was 65.5% reduction in median PASI in Group A versus 91% reduction in Group B [Table - 2]. Moderate psoriasis has also been defined by PASI >7.
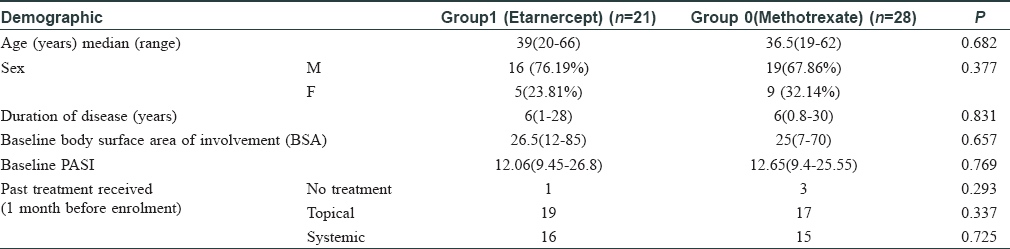

Both PGA and IGA scores significantly improved within both groups (P ≤ 0.01), but they were comparable between the two groups (P = 0.43 and 0.46, respectively) [Figure - 1] and [Figure - 2].
 |
| Figure 1: Comparison of median patient global assessment scores in the 2 study groups |
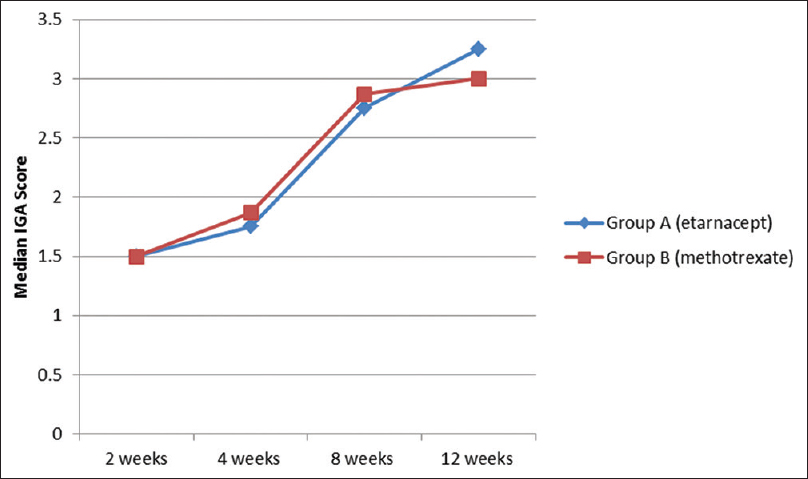 |
| Figure 2: Comparison of median investigator global assessment scores in the 2 study groups |
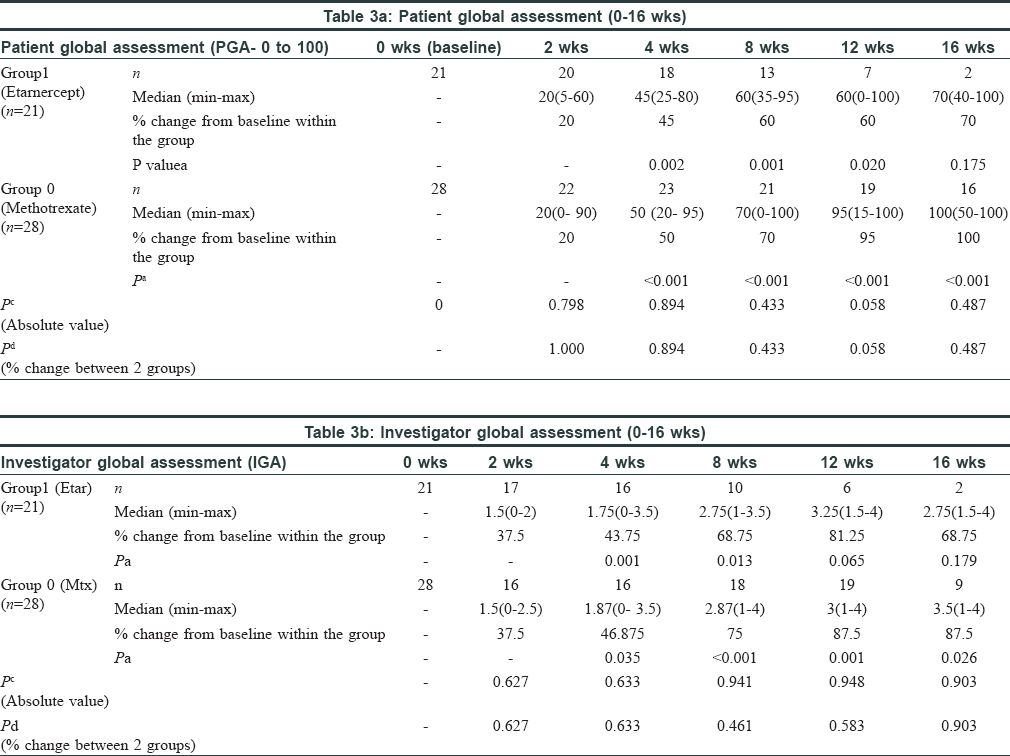
Comparisons were made for PGA and IGA categorically for each follow up visit within the groups as well as between the groups. The comparative tables are given below [Table - 3]a and [Table - 3]b.
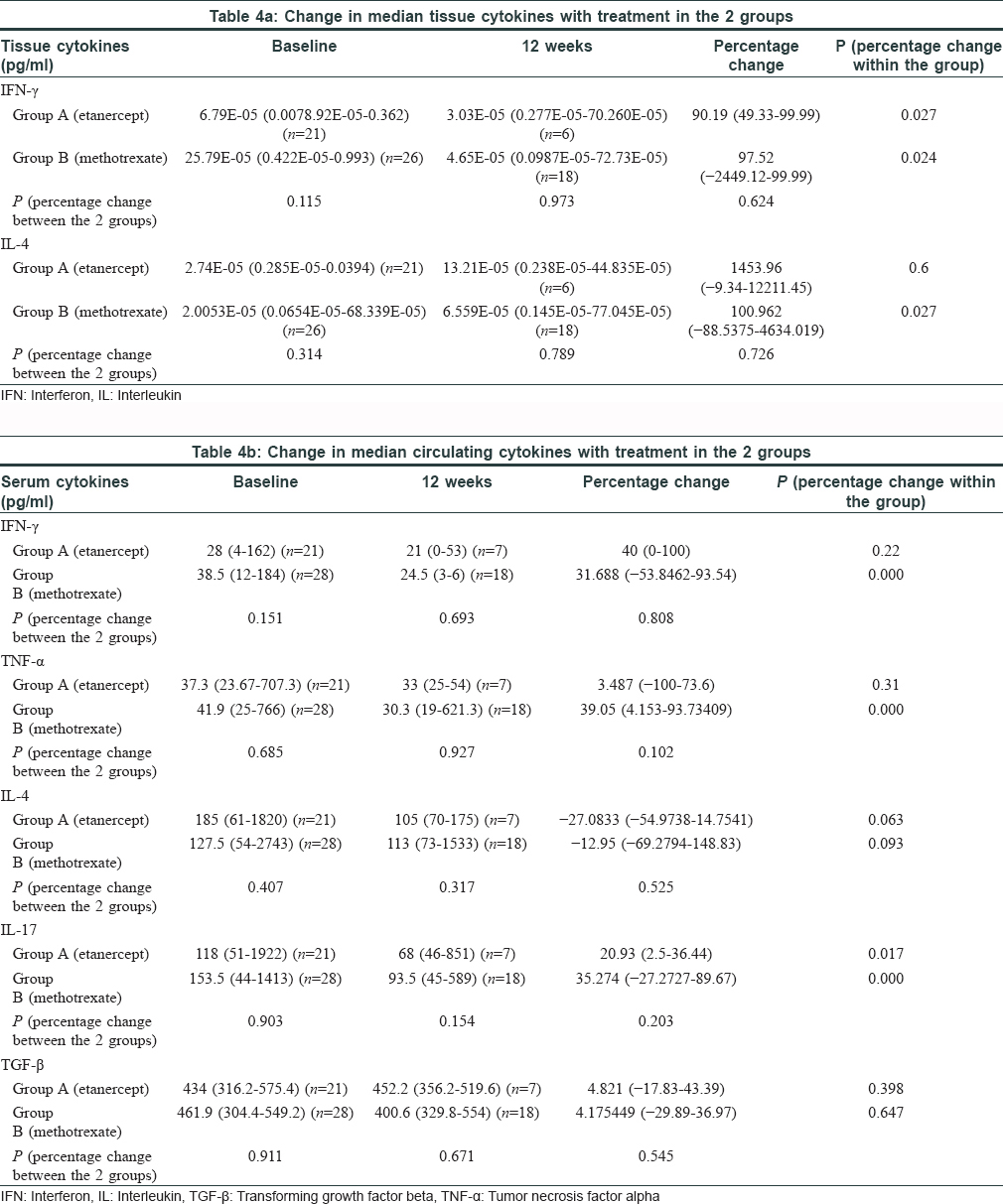
Median tissue IFN-γ level reduced by 90.2% in Group A (P = 0.027) and by 97.5% in Group B (P = 0.024) at week12. IL-4 level showed a significant rise from baseline in the methotrexate group (P = 0.027). Change in both the tissue cytokines between the two groups was comparable [Table - 4]a. A significant decline was observed in serum IL-17, IFN-γ and TNF-α in the methotrexate group and in IL-17 level in the etanercept group, but inter-group analysis showed it to be comparable between the two groups [Table - 4]b.
Adverse events like uneasiness, nausea and vomiting were only observed in Group B. No other significant adverse events were noted either group.
Both methotrexate and etanercept are effective in psoriasis. Methotrexate showed PASI 75 in 35%–40% patients after 16 to 24 weeks of treatment[4] while etanercept showed PASI 75 in 49%–57% patients at week 12 in the 50 mg twice weekly group.[5] A systematic review of trials revealed that biologicals have a slightly higher likelihood of achieving therapeutic response compared to nonbiologic systemic agents. Primary outcome measure was comparison of mean percentage reduction in PASI at week 8 between two groups. This has been mentioned in results. PASI 50, 75 and 90 in the 2 groups which were secondary outcome parameters, have been mentioned in the discussion as mentioning this in the result also increases the word count. Mentioning the % change as per IGA/PGA was making us extend beyond the word limit, hence, data has been provided in form of graphs.. As very few patients received etanercept after 8 weeks, we were unable to compare them at 12th week.
Only a few studies have analyzed the modifications of T cell responses in psoriasis post-treatment. They depicted the failure of conventional agents to influence Th1/Th2 balance.[3] Quaglino et al. reported significant reversal of Th1/Th17 activation by etanercept and concomitant upregulation of Th2/T-reg subsets, which correlated well with clinical response.[6] We observed a similar pattern of cytokine change with both etanercept and methotrexate. There was a significant rise in tissue IL-4 with methotrexate and decline in IFN-γ with both modalities. There was comparable decline in serum IFN-γ (P = 0.808), TNF-α (P = 0.102), IL-17 (P = 0.203) and IL-4 (P = 0.525) in both groups at week 12.
A small sample size may be the reason for no statistical difference in the 2 groups. Lack of randomization due to logistic reasons was another limitation. Moreover a 12 week period of follow-up may be inadequate to assess absence of new TB cases/reactivation of TB with the biological agent.
Sample size calculation
Taking percentage of patients achieving PASI 75 at the end of 12 weeks as 35.5% for methotrexate and 49% for etanercept (50mg biweekly) (as per previous studies), and a power of 80% and p value of 0.05, the sample size was calculated to be 294. Considering for allowance for losses of 20%, the required sample size came as 368.
However, because of limitation of costs of drug (etanercept- Rs.14,350/-per vial of 50 mg drug), and a fixed number of vials provided by the funding agency, we were able to recruit a fixed number of patients. Moreover it was a preliminary, non-randomized study.
Both etanercept and methotrexate showed good and comparable clinical outcomes, and an overall better and faster response was seen with methotrexate. A similar trend in skin and serum cytokine profile was observed. Hence, methotrexate remains an effective and comparatively inexpensive treatment for moderate-to-severe plaque psoriasis, whereas etanercept could be a better alternative for recalcitrant patients.
Acknowledgement
We thank Preeti Sharma for helping with serum cytokine analysis, Dr. M Kalaivani for statistical analysis, Dr. Kanika Sahni for helping in the preparation of project for ethics committee approval and Dr. Rubina Jassi and Dr. Deepika Yadav for doing the investigator global assessment scores.
Financial support and sponsorship
M/s Pfizer funded the study and provided 361 injections of Enbrel 50 mg each.
Conflicts of interest
It was an investigator-initiated trial in which the funding agency (M/S Pfizer) had no interference in the design, conduct and analysis of the study.[7]
| 1. |
Huerta C, Rivero E, Rodríguez LA. Incidence and risk factors for psoriasis in the general population. Arch Dermatol 2007;143:1559-65.
[Google Scholar]
|
| 2. |
Nickoloff BJ. Cracking the cytokine code in psoriasis. Nat Med 2007;13:242-4.
[Google Scholar]
|
| 3. |
Caproni M, Antiga E, Melani L, Volpi W, Del Bianco E, Fabbri P. Serum levels of IL-17 and IL-22 are reduced by etanercept, but not by acitretin, in patients with psoriasis: A randomized-controlled trial. J Clin Immunol 2009;29:210-4.
[Google Scholar]
|
| 4. |
Saurat JH, Stingl G, Dubertret L, Papp K, Langley RG, Ortonne JP, et al. Efficacy and safety results from the randomized controlled comparative study of adalimumab vs. methotrexate vs. placebo in patients with psoriasis (CHAMPION). Br J Dermatol 2008;158:558-66.
[Google Scholar]
|
| 5. |
Papp KA, Tyring S, Lahfa M, Prinz J, Griffiths CE, Nakanishi AM, et al. A global phase III randomized controlled trial of etanercept in psoriasis: Safety, efficacy, and effect of dose reduction. Br J Dermatol 2005;152:1304-12.
[Google Scholar]
|
| 6. |
Quaglino P, Ortoncelli M, Comessatti A, Ponti R, Novelli M, Bergallo M et al. Circulating CD4+ CD25brightFOXP3+ T cells are up-regulated by biological therapies and correlate with the clinical response in psoriasis patients. Dermatology 2009;219:250-8.
[Google Scholar]
|
| 7. |
Llamas-Velasco M, de la Cueva P, Notario J, Mart�nez-Pilar L, Martorell A, Moreno-Ram�rez D. Moderate psoriasis: a proposed definition. Actas Dermosifiliogr 2017;108:911-17.
[Google Scholar]
|
Fulltext Views
3,915
PDF downloads
2,812





