Translate this page into:
A solitary cutaneous metastasis from colon adenocarcinoma: Dermoscopic and confocal microscopy features
2 Department of Oncology, University Hospital ASST Spedali Civili of Brescia, Brescia, Italy
Correspondence Address:
Marta Fusano
Department of Dermatology, University Hospital Asst Spedali Civili of Brescia, Piazzale Spedali Civili 1, 25123 Brescia
Italy
| How to cite this article: Manganoni AM, Fusano M, Pavoni L, Pizzati L, Consoli F, Venturini M, Zanca A, Calzavara-Pinton P. A solitary cutaneous metastasis from colon adenocarcinoma: Dermoscopic and confocal microscopy features. Indian J Dermatol Venereol Leprol 2018;84:589-591 |
Sir,
A limited number of studies have reported cutaneous metastases in colorectal adenocarcinoma patients. Here, we report a case of a solitary cutaneous metastasis of colorectal adenocarcinoma in a 51-year-old man. We emphasize the importance of dermatologic evaluation in oncologic patients that show a persistent cutaneous plaque or nodule. and stress that combining dermoscopy and reflectance confocal microscopy improves diagnostic accuracy for equivocal lesions.
We report the case of a 51-year-old man with an adenocarcinoma of the colon associated with focal nodal metastases (T3 N2 M0). The patient underwent right hemicolectomy followed by adjuvant chemotherapy. Oncological outcome after two years was uneventful until September 2015, when the patient was referred to our department for a fast growing plaque on the left parietal side of the scalp. Clinically, the plaque appeared as a solitary well-demarcated skin-colored lesion of 1.5 cm diameter, leading to an area of alopecia, without any sign of inflammation [Figure - 1]. The lesion clinically appeared as a subcutaneous or intradermal nodule, resembling a basal cell carcinoma, a merkel cell carcinoma or a benign lesion such as lipoma or a connective tissue lesion.
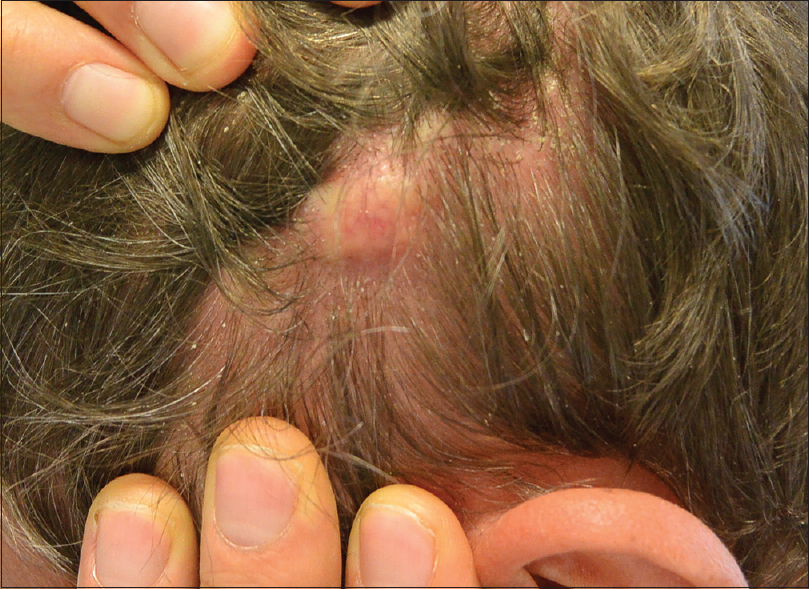 |
| Figure 1: Solitary skin-colored nodule on physical examination |
On dermoscopy, performed with an oil immersion nonpolarized dermatoscope, the main feature observed was the irregularity and polymorphism of vessels; we noted dotted, hairpin and looped vessels at the periphery of the lesion while we identified irregular, linear and antler-like vascular pattern in the center [Figure - 2]. These findings appeared really uncommon for a basal cell carcinoma; and the, peculiar pattern of angiogenesis led us to suspect a malignant lesion.
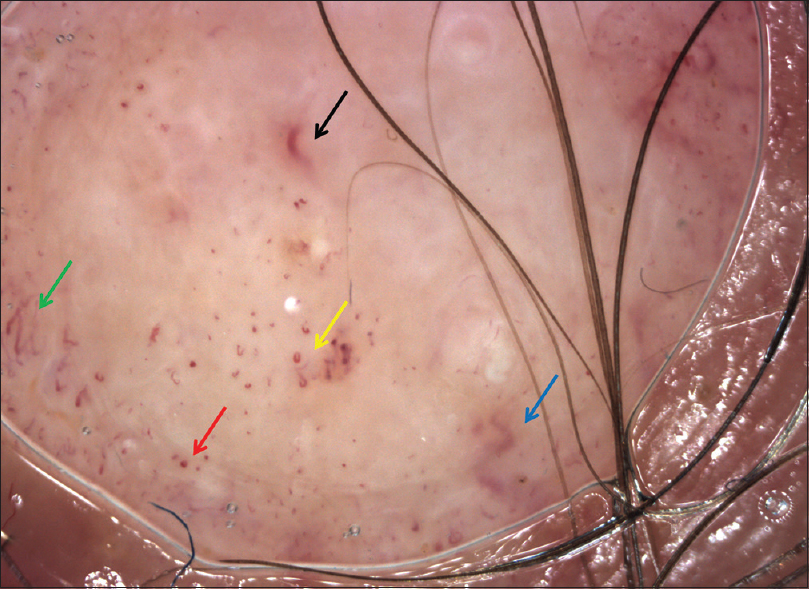 |
| Figure 2: Dermoscopy showed dotted (red arrow), hairpin (green arrow) and looped vessels (yellow arrow) on periphery lesion, irregular linear (black arrow) and antler-like vessels (blue arrow) in the middle. Image obtained using videodermoscopy digital systems (Vidix, ×500) |
Reflectance confocal microscopy at the dermal–epidermal junction showed aggregates of atypical cells with different sizes forming nests that were not homogeneous in reflectivity [Figure - 3]. Differentiating this lesion from basal cell carcinoma was made possible by the absence of typical findings (such as elongated polarized basaloid nuclei at the epidermal level or tortuous and ecstatic blood vessels around the nests).
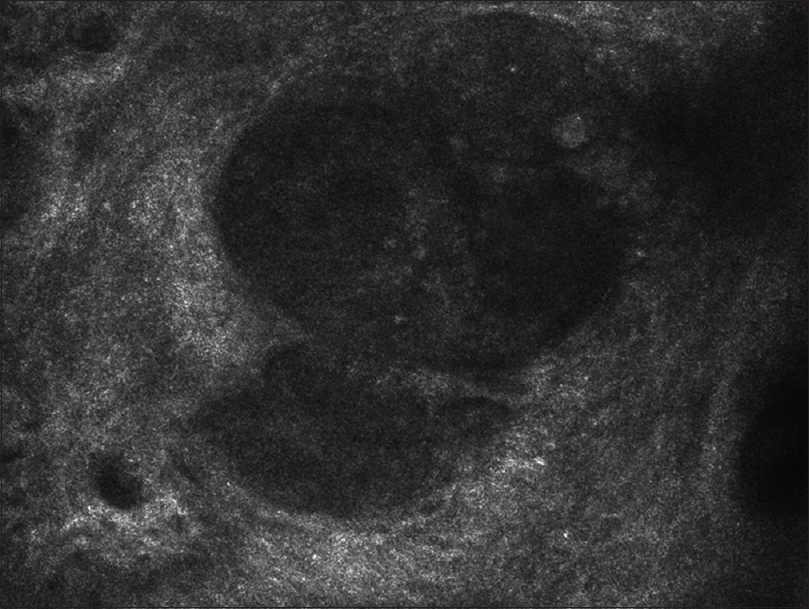 |
| Figure 3: Reflectance confocal microscopy: Aggregates of atypical cells at the dermal-epidermal junction |
Complete excision of the plaque with histopathological examination was performed. Histopathology revealed an atypical glandular pattern, suggestive of a poorly differentiated adenocarcinoma [Figure - 4]a. Immunohistochemistry showed high positivity for caudal type homeobox transcription factor 2, hence, the diagnosis of cutaneous metastases from colonic carcinoma was evident [Figure - 4]b.
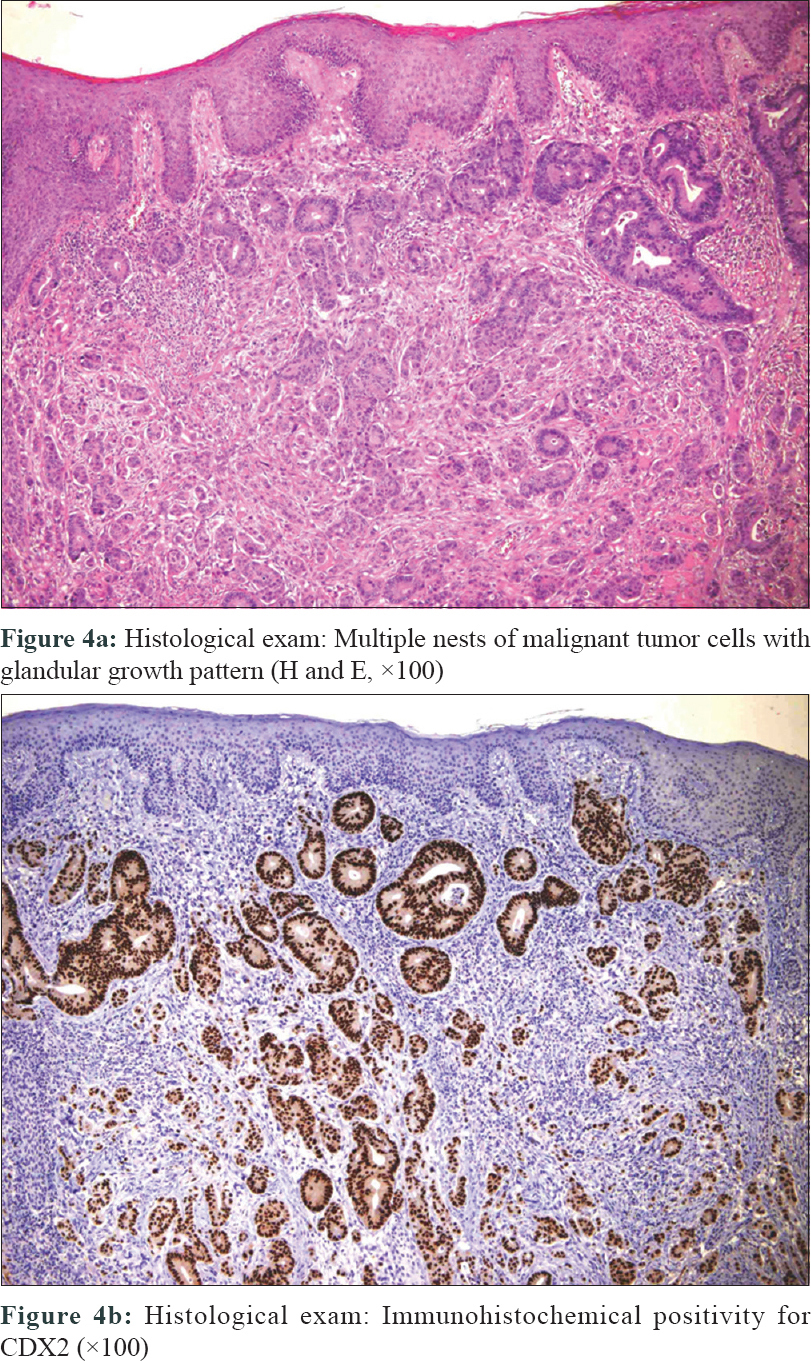 |
| Figure 4: |
The patient underwent total-body computed tomography scan to restage the tumoral disease, Multiple small nodular bilateral lung lesions and a singular lesion on segment IV of the liver were found. Segmental resection of the liver was performed, following which chemotherapy was started. Few months later, brain metastases were found at the follow-up and it was decided to continue palliative therapies alone.
Cutaneous metastasis is a rare clinical finding that usually represents the recurrence of primary visceral tumor with disseminated disease. Cutaneous metastases clinically appears as a subcutaneous or intradermal nodule, a nodulocystic lesion, an ulceration, a cellulitis-like lesion or fibrotic processes; it can be misdiagnosed as it resembles simple cysts, lipomas, merkel cell carcinoma, neurofibromas or other benign connective tissue lesions. In the case of complex lesions, an interdisciplinary approach that combines dermatology, oncology and surgery can provide optimal care for the patient.[1]
There is very less data on dermoscopic findings in skin metastases in published literature. Chernoff and Marghoob described irregular vascular structures as the most common dermoscopic finding in 20 skin secondaries from different visceral cancers, whereas Ito et al. reported dotted, irregular linear and looped vessels in a case of peristomal metastasis.[2] Otherwise, it is not possible to demonstrate the phenomena of cutaneous metastases from visceral neoplasia using a videodermoscope. In fact, dermoscopy in our case did not reveal specific patterns that could have helped in clinical diagnosis. However, it can suggest the necessity of further diagnostic tools particularly when vessels, assessed at a high magnification (e.g. 500× magnification), show irregularity and polymorphism and are observed close to the tumor edges. Pizzichetta et al. noted that, in metastatic melanoma, the disposition of vascular structure could be a diagnostic indicator of metastatic tumor.[3] In any case, a biopsy is needed for the diagnosis even when similar dermoscopic findings are present.
In literature, the applications and findings of reflectance confocal microscopy are described in benign and pathological skin involvement, but few such reports exist on cutaneous metastases.[4],[5] In our case, we excluded the diagnosis of basal cell carcinoma thanks to the absence of typical basoloid cells and vascular pattern; the presence of atypical unknown cells led us to suspect other tumoral disease.
A close correlation between the features observed dermoscopically and the histological features of excised skin lesion is not seen. The examination of vascular pattern is of particular interest in diagnosing nonpigmented lesions, where the vascular structures could be a diagnostic indicator and are often the only clues that suggest the presence of atypia. Metastatic melanoma may mimic other lesions, such as cutaneous metastases of visceral tumor, and their differentiation is critical.[6] Especially when the primary cancer is unknown, immunohistochemical studies may be helpful in arriving at the correct diagnosis. The skin tumors were indicative of progression of the primary tumor.
This case highlights the complications of disease process and stresses on additional techniques that can be used to detect occult disease in oncologic patients.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form, the patient has given his consent for his images and other clinical information to be reported in the journal. The patient understands that name and initials will not be published and due efforts will be made to conceal identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
| 1. |
Manganoni AM, Farisoglio C, Ferrari V, Zaniboni A, Beretta G, Meriggi F, et al. Multidisciplinary team-working indicators of good practice in the clinical management of EGFR-inhibitor dermatologic toxicities. Ann Surg Oncol 2009;16:224-5.
[Google Scholar]
|
| 2. |
Ito T, Yoshida Y, Yamada N, Furue M, Yamamoto O. Dermoscopy of peristomal polyps and metastasis of colon cancer. Acta Derm Venereol 2014;94:96-7.
[Google Scholar]
|
| 3. |
Pizzichetta MA, Canzonieri V, Massarut S, Baresic T, Borsatti E, Menzies SW, et al. Pitfalls in the dermoscopic diagnosis of amelanotic melanoma. J Am Acad Dermatol 2010;62:893-4.
[Google Scholar]
|
| 4. |
González S, Gilaberte-Calzada Y.In vivo reflectance-mode confocal microscopy in clinical dermatology and cosmetology. Int J Cosmet Sci 2008;30:1-7.
[Google Scholar]
|
| 5. |
Longo C, Benati E, Borsari S, Bombonato C, Pampena R, Moscarella E, et al. Merkel cell carcinoma: Morphologic aspects on reflectance confocal microscopy. J Eur Acad Dermatol Venereol 2017;31:e480-1.
[Google Scholar]
|
| 6. |
Rubegni P, Lamberti A, Mandato F, Perotti R, Fimiani M. Dermoscopic patterns of cutaneous melanoma metastases. Int J Dermatol 2014;53:404-12.
[Google Scholar]
|
Fulltext Views
2,725
PDF downloads
1,188





