Translate this page into:
A study of serum vascular endothelial growth factor in infantile hemangiomas
2 Department of Biochemistry, Lady Hardinge Medical College and Associated Hospitals, New Delhi, India
Correspondence Address:
Saurabh Mittal
Department of Dermatology and STD, Lady Hardinge Medical College and Associated Hospitals, Shahid Bhagat Singh Marg, New Delhi - 110 001
India
| How to cite this article: Paria S, Mendiratta V, Chander R, Jain A, Mittal S. A study of serum vascular endothelial growth factor in infantile hemangiomas. Indian J Dermatol Venereol Leprol 2019;85:65-68 |
Abstract
Background: Though infantile hemangiomas are the most common benign tumor of infancy, their etiopathogenesis is not fully understood. Some studies report a diagnostic role for vascular endothelial growth factor (VEGF), but such studies are lacking from India.
Aims: To study the clinicoepidemiological profile of infantile hemangiomas, to estimate and compare the serum levels of VEGF in infantile hemangiomas and controls, and to determine correlations between serum levels of VEGF and growth characteristics of infantile hemangiomas.
Methods: A hospital-based, cross-sectional study was carried out on 30 clinically diagnosed cases of infantile hemangioma and 30 controls presenting with other disorders. VEGF levels were recorded for both cases and controls by the sandwich enzyme-linked immunosorbent assay (ELISA) technique. Results were analyzed using SPSS version 20.0, and their significance determined using appropriate tests.
Results: Mean serum VEGF level in the cases was 216.8 ± 49.2 pg/ml while in the control group it was 115.1 ± 43.1 pg/ml (P < 0.0001). There were no statistically significant correlations between serum VEGF levels and sex or size, phase of growth, morphological variants or ulceration of lesions.
Limitations: Our sample was not large enough to draw clinically applicable conclusions. An adequate sample size could not be achieved because of low incidence of the disease, and resource and time constraints.
Conclusions: The mean value of serum VEGF in the study group was significantly higher than that in the control group, suggesting that serum VEGF can serve as a diagnostic marker of infantile hemangiomas. Mean serum VEGF was higher in proliferative lesions than in involuting lesions, indicating that it may also be useful as a prognostic serological marker in cases of infantile hemangioma.
Introduction
Infantile hemangioma is the most common benign tumor of infancy affecting 5–10% of infants by the age of 1 year[1] and can be associated with complications in as high as 40% of cases.[2] The etiopathogenesis of infantile hemangiomas is still not fully understood, but an impaired balance between proangiogenic and antiangiogenic factors has been implicated in the development of hemangiomas. Some studies also report a diagnostic role for vascular endothelial growth factor (VEGF) in differentiating hemangiomas from vascular malformations.[3] Studies that describe the clinicoepidemiological features of infantile hemangiomas and the putative role of VEGF in hemangioma proliferation are lacking from India.
The aims of this study were to study the clinicoepidemiological profile of infantile hemangiomas, to compare serum levels of VEGF between cases with infantile hemangiomas and controls, and to determine correlations between serum levels of VEGF and growth characteristics of infantile hemangiomas.
Methods
This was a hospital-based, cross-sectional, case-control study, and included all clinically diagnosed cases of infantile hemangiomas presenting to the Dermatology Department. The study was approved by the Institutional Ethics Committee.
Children of all groups and both sexes who were clinically diagnosed with superficial and mixed infantile hemangiomas of size >2 cm2 and who had not received any treatment in the last 4 weeks were enrolled. Age- and sex-matched children with other conditions were enrolled as controls. Children with deep or systemic infantile hemangiomas, infantile hemangiomas in the involution stage, vascular malformations, macular and/or telangiectatic lesions and those with known malignancies or other systemic abnormalities were excluded.
The growth phase of each lesion was determined by the clinical criteria listed in [Table - 1]. Growth phases of hemangiomas were also differentiated based on lesional color, a bright red corresponding to the proliferative phase [Figure - 1], red to gray to the involuting phase [Figure - 2] and a pale color to the ‘involuted phase’ of hemangiomas.
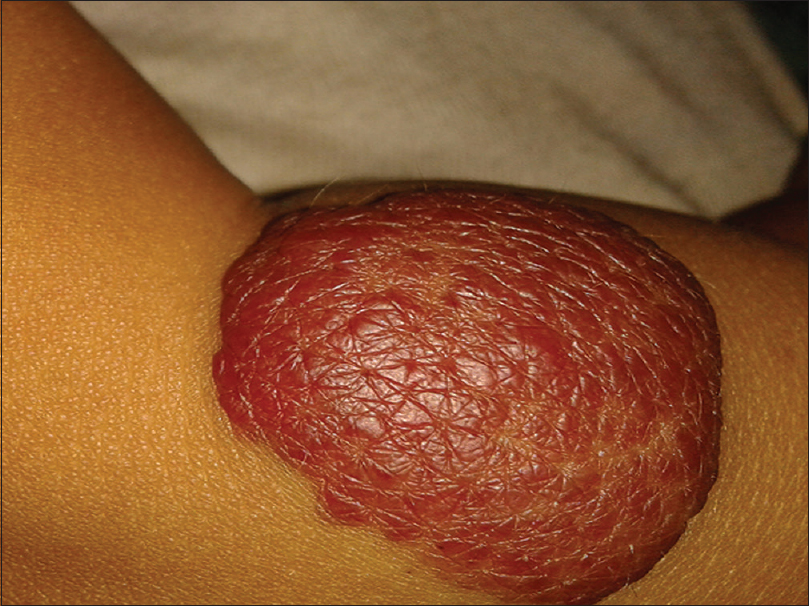 |
| Figure 1: Infantile hemangioma: bright red color indicating the proliferating phase |
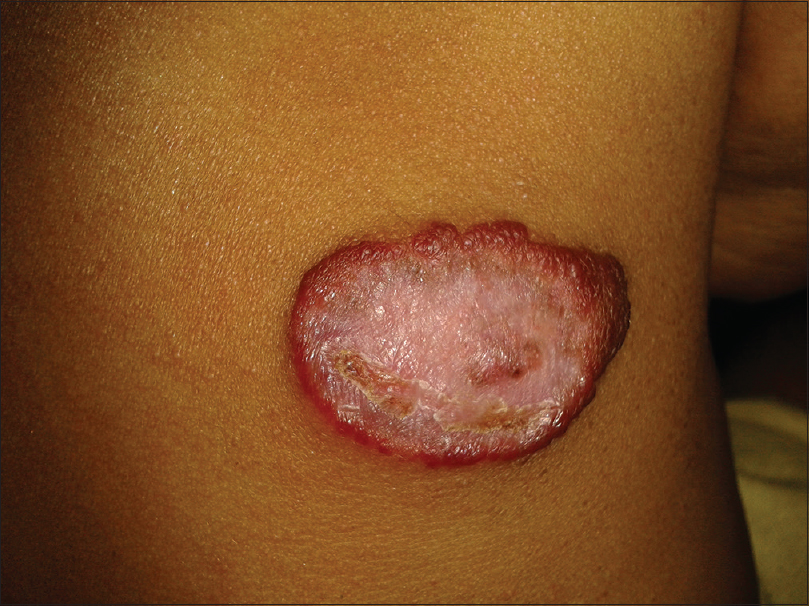 |
| Figure 2: Infantile hemangioma: dull red-to-gray color of an involuting lesion |
To correlate VEGF levels with the size of lesions, cases were divided into three groups based on the surface area of the largest lesion: those with reference lesion sized <4 cm2, 4-6 cm2 and >6 cm2.
VEGF levels were determined in cases and controls by the sandwich enzyme-linked immunosorbent assay (ELISA) technique using the human VEGF-A BioLISA kit by DIACLONE. All findings including demographic data, clinical findings and lab results were recorded on a preset proforma. Results were analyzed using SPSS version 20.0 and significance of the results was calculated using appropriate tests such as student's ‘t’ test, Mann Whitney U test, ANOVA test, Pearson correlation coefficient and correlation analysis.
Results
A total of 30 cases and 30 controls were included. Age ranged from 4 days to 24 months in the study group with a mean age of 6.12 months while mean age in the control group was 5.4 months. A majority (36.4%) of the infants were 0–3 months old, and females outnumbered males by a ratio of 1.73:1. Ninety percent of the babies had been delivered vaginally, and 63.3% of the mothers were primigravida. Twenty percent of the mothers gave a history of fever during pregnancy. A total of 16.7% of the mothers had a history of drug intake during pregnancy in the form of oral contraceptives (6.7%), antithyroid drugs (6.7%), and antitubercular treatment (3.3%). Seven babies in the study group were preterm. There was no family history of infantile hemangiomas in any of the cases.
Most (53.3%) of the babies had lesion onset during the first week of life, but it was only during the 2nd month that a majority (43.3%) of the lesions began to proliferate. Sixty percent of cases had single lesions, while the rest had multiple lesions, yielding an average of 1.67 lesions per child. Seventy percent of cases had a superficial variant while the others had mixed type of infantile hemangiomas. The most common site affected was the face (50%), with the forehead being the most common location thereon (5 cases); the lower limbs and the genitalia were the least affected sites (3.3% each). Eighty percent of cases had hemangiomas in the growing phase and 53.3% of the cases had complications, with bleeding being the most common in 26.67% of cases.
Mean serum VEGF level in the cases was 216.8 ± 49.2 pg/ml whereas in the control group it was 115.1 ± 43.1 pg/ml (P < 0.0001). There was no significant difference in serum VEGF levels between the sexes.
Mean serum VEGF levels were also analysed with respect to various infantile hemangioma characteristics [Table - 2]. The mean VEGF levels in proliferating lesions was 226.04 ± 39.78 pg/ml compared to 180 ± 68.77 pg/ml in involuting lesions, though this difference was not statistically significant (P = 0.131). VEGF levels in those with non-ulcerated lesions (219.78 ± 49.5 pg/ml) were found to be higher than in those with ulceration (207.5 ± 55.0 pg/ml); this difference too was not statistically significant. Mean serum VEGF was higher (347 ± 17.35 pg/ml) in superficial lesions than in the mixed variant (118 ± 11.8 pg/ml).
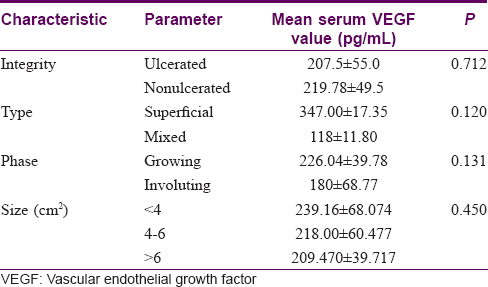
VEGF levels were found to be inversely proportional to the size, although this difference was also not statistically significant.
Discussion
The word hemangioma is derived from the Greek ‘haima’ meaning blood and the condition was first documented by Liston in 1843. Infantile hemangiomas occur more frequently in female infants,[4] as in our study. Nearly, 70% of our cases had their lesions in the head and neck region. Finn et al. in their study too found that the head and neck are the most commonly affected sites (approximately 60% of cases).[5]
There are several hypotheses regarding the etiopathogenesis of infantile hemangiomas which are not mutually exclusive; most include a role for VEGF in the abnormal proliferation of the endothelial cells. Infantile hemangiomas share many immunohistochemical markers with placental microvessels. The placenta also produces very high levels of the proangiogenic cytokine VEGF; as a protective mechanism against uncontrolled angiogenesis, a soluble VEGF receptor sFlt-1 is also produced by the placenta. Postpartum, the connection to the placenta and subsequently sFlt-1 is removed, which may lead to the unchecked growth of tissues responsive to VEGF, such as infantile hemangiomas.[6],[7] Another theory proposes that infantile hemangiomas may be a result of abnormal proliferation of endothelial progenitor cells, which are bone marrow-derived VEGF receptor (VEGFR-2)-positive pluripotent cells.[8] Missense mutations in genes encoding VEGFR-2 and TEM-8 have been identified in a subset of infantile hemangiomas, which may be responsible for their constitutive activation and resultant hemangioma endothelial cell proliferation.[9] VEGF, a member of the platelet-derived growth factor (PDGF) family, was initially isolated as a tumor-derived factor that increases vascular permeability and subsequently as a mitogen having high specificity for endothelial cells.[10],[11]
There is a paucity of case-control studies of VEGF levels in infantile hemangiomas. In two separate studies by Przewratil et al., it was found that the local serum levels of VEGF were significantly higher than in the peripheral blood, and that serum VEGF levels differed significantly between those with infantile hemangiomas and controls, while there was no significant difference between the sexes.[3],[12] Our study confirms these findings. We too noted that the VEGF levels of those in the growth phase were higher than those in the involutional phase, supporting a possible etiopathogenetic role of VEGF in infantile hemangiomas and its role in the growth of these lesions. However, in both our study and the one by Przewratil et al.[12] the differences were not significant.
We found that VEGF levels in those with non-ulcerated lesions were higher than in those with ulceration, although the difference was not statistically significant. It is possible that VEGF levels peak during the growth phase which is achieved some time before ulceration sets in, leading to this difference in levels, though there are no other reports on this aspect.
The mean serum levels of VEGF was also higher in superficial lesions than in the the mixed variant, perhaps because most of the superficial lesions (70%) were in the growth phase, compared to only 13.3% of the mixed variety. Because there were more than one hemangioma in most of the study cases, it was difficult to correlate VEGF levels with the size of lesions. Contrary to the expected, it was seen that the VEGF levels were inversely proportional to the size of the reference lesion, although the difference was not statistically significant. Studying the volume relation, rather than area, with VEGF levels may have yielded different results but this could not be done due to resource constraints. Of note, Przewratil et al. showed a positive correlation between VEGF levels and the volume of hemangiomas, though the results again were not statistically significant.[3]
Despite its frequency, there are no definite markers for the diagnosis and prognosis of infantile hemangioma. Based on its role in the pathogenesis of infantile hemangiomas, serum and local VEGF levels might fill this gap. Investigative modalities currently available are limited by factors such as radiation exposure to infants, cost, and their limited capacity to delineate lesions. VEGF levels, on the other hand, are easier to determine. We conclude that serum VEGF levels may be related more to active angiogenesis than to other factors, and by serially measuring its levels it may be possible to gauge the progression and natural course of these lesions. VEGF measurements may also help differentiate between infantile hemangiomas and vascular malformations.
Limitations
The sample size in this study was only enough to draw preliminary conclusions; a larger sample size is needed to arrive at clinically meaningful conclusions. We could not include a larger sample because of low incidence of the disease, and resource and time constraints. Correlations between the volume of infantile hemangiomas and VEGF levels could not be looked at due to a lack of resources for lesional volume measurements.
Conclusions
There are very few published studies on serum VEGF levels in cases of cutaneous infantile hemangioma. To our knowledge, no such study has been conducted in this part of the world. Based on our study and published literature, we recommend that further studies be conducted, including larger numbers of cases, to study the levels of serum VEGF in cases of infantile hemangioma and their correlations with various parameters.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
| 1. |
Drolet BA, Esterly NB, Frieden IJ. Hemangiomas in children. N Engl J Med 1999;341:173-81.
[Google Scholar]
|
| 2. |
Chiller KG, Passaro D, Frieden IJ. Hemangiomas of infancy: Clinical characteristics, morphologic subtypes, and their relationship to race, ethnicity, and sex. Arch Dermatol 2002;138:1567-76.
[Google Scholar]
|
| 3. |
Przewratil P, Sitkiewicz A, Andrzejewska E. Soluble receptors for vascular endothelial growth factor (sVEGFR1/sVEGFR2) in infantile hemangioma. Growth Factors 2010;28:417-25.
[Google Scholar]
|
| 4. |
Drolet BA, Swanson EA, Frieden IJ, Hemangioma Investigator Group. Infantile hemangiomas: An emerging health issue linked to an increased rate of low birth weight infants. J Pediatr 2008;153:712-5, 715.e1.
[Google Scholar]
|
| 5. |
Finn MC, Glowacki J, Mulliken JB. Congenital vascular lesions: Clinical application of a new classification. J Pediatr Surg 1983;18:894-900.
[Google Scholar]
|
| 6. |
Banks RE, Forbes MA, Searles J, Pappin D, Canas B, Rahman D, et al. Evidence for the existence of a novel pregnancy-associated soluble variant of the vascular endothelial growth factor receptor, Flt-1. Mol Hum Reprod 1998;4:377-86.
[Google Scholar]
|
| 7. |
Hornig C, Barleon B, Ahmad S, Vuorela P, Ahmed A, Weich HA, et al. Release and complex formation of soluble VEGFR-1 from endothelial cells and biological fluids. Lab Invest 2000;80:443-54.
[Google Scholar]
|
| 8. |
Yu Y, Flint AF, Mulliken JB, Wu JK, Bischoff J. Endothelial progenitor cells in infantile hemangioma. Blood 2004;103:1373-5.
[Google Scholar]
|
| 9. |
Jinnin M, Medici D, Park L, Limaye N, Liu Y, Boscolo E, et al. Suppressed NFAT-dependent VEGFR1 expression and constitutive VEGFR2 signaling in infantile hemangioma. Nat Med 2008;14:1236-46.
[Google Scholar]
|
| 10. |
Senger DR, Connolly DT, Van de Water L, Feder J, Dvorak HF. Purification and NH2-terminal amino acid sequence of guinea pig tumor-secreted vascular permeability factor. Cancer Res 1990;50:1774-8.
[Google Scholar]
|
| 11. |
Keck PJ, Hauser SD, Krivi G, Sanzo K, Warren T, Feder J, et al. Vascular permeability factor, an endothelial cell mitogen related to PDGF. Science 1989;246:1309-12.
[Google Scholar]
|
| 12. |
Przewratil P, Sitkiewicz A, Andrzejewska E. Local serum levels of vascular endothelial growth factor in infantile hemangioma: Intriguing mechanism of endothelial growth. Cytokine 2010;49:141-7.
[Google Scholar]
|
Fulltext Views
3,087
PDF downloads
2,800





