Translate this page into:
Anatomical segmentations in all forms of vitiligo: A new dimension to the etiopathogenesis
Correspondence Address:
Venkata Ratnam Attili
Visakha Institute of Skin and Allergy, Marripalem, Visakhapatnam - 530 018, Andhra Pradesh
India
| How to cite this article: Attili VR, Attili SK. Anatomical segmentations in all forms of vitiligo: A new dimension to the etiopathogenesis. Indian J Dermatol Venereol Leprol 2016;82:379-388 |
Abstract
Background: We have reported segmented lesions in acral vitiligo as well as in generalized vitiligo and thereby proposed somatic mosaicism as a predisposing feature in all forms of vitiligo. This study is a further attempt to characterize and understand such segmented lesions by screening a large series of patients. Methods: We searched our electronic archives (from 2002 to 2014) and identified/reviewed the photos of 615 vitiligo patients inclusive of all clinical types. Over 3500 photographs were screened for patterns that were repeatedly seen in two or more patients and a composite picture of these were marked on a body map. Results: Similar unilateral/bilateral segmented lesions were identified among all forms of vitiligo during relatively stable phases of the disease. These appeared to be related to small and large anatomical divisions of the body. In rapidly evolving disease on the trunk, the lesions conformed to Blaschkoid patterns. Several instances of stable mirror image lesions, symmetric incremental progressions and regressions were also recorded. Limitations: These are observations of a retrospective, single-center review which need to be substantiated further in larger prospective studies. Conclusion: Similar unilateral/bilateral segmented patterns delineating major/minor anatomical divisions of the body may indicate a preexisting developmental defect (such as mosaicism).Introduction
Varied clinical patterns and divergent pathogenic factors proposed in vitiligo[1],[2],[3],[4],[5] raise a pertinent question: is it a single disease presenting in many clinical forms or a clinical syndrome of asymptomatic macular depigmentation resulting from multiple pathogenic events?[6] A global conference recommended primary classification of vitiligo as segmental vitiligo and non-segmental vitiligo implying different evolutionary patterns and pathogenesis.[7] Nevertheless, some authors prefer to classify vitiligo as unilateral or bilateral or simply identify the clinical variants without attempting a classification.[8],[9] There are several drawbacks with the segmental/non-segmental bifurcation. There has been no clear definition of “segment” which can be of different phenotypes and distribution patterns, unilateral, bilateral and pleuri-segmental.[10],[11],[13] The acceptance of mixed vitiligo, a combination of segmental and non-segmental forms contradicts the presumed clinical dichotomy and divergent pathogenesis of the two forms.[14],[15] Moreover, we have recently reported “segmented” and mirror image bilateral lesions in acral vitiligo, as well as in generalized vitiligo thus calling into question the basis for classification of vitiligo into segmental and non-segmental forms.[16],[17] Instead, we have proposed that such segmentations are a possible result of somatic mosaicism. The objective of this study was to further evaluate and characterize the nature of such segmentations in a retrospective review of a large number of vitiligo cases of all clinical types.
Methods
It has been a standard practice in our institute since 2002 to photograph all vitiligo lesions, initially and also at all follow-up visits, to assess the treatment response. Vitiligo lesions were classified in accordance with the currently accepted classification into segmental (151) or non-segmental (464).[13] Among the latter, two major subdivisions were identified: generalized vitiligo (131) with dominant trunk involvement (generalized non-acrofacial vitiligo) and acral vitiligo (193) which essentially involved the peripheral body parts.[17] The rest were grouped as “focal lesions limited to one anatomical area” (83) or “multifocal lesions involving more than one area, but with no bilateral symmetry” (57). Case histories and photographs (3500) of 615 vitiligo cases registered over a period of 12 years were reviewed. Lesions were mapped and screened for repetitive unilateral or bilateral segmentations. When a segmented pattern was seen to be repeated in two or more patients, it was marked as a template to compare with others.
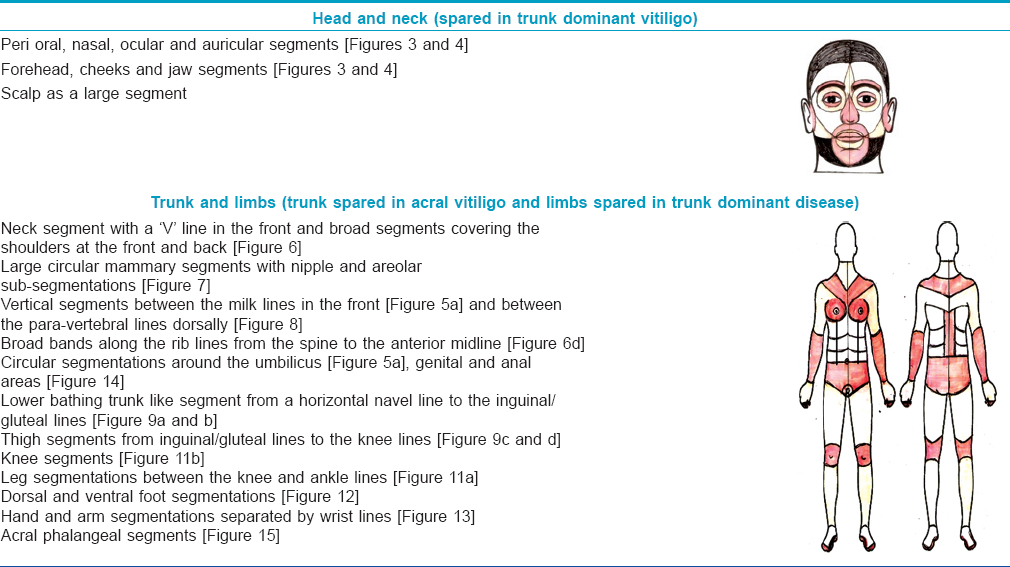
Results
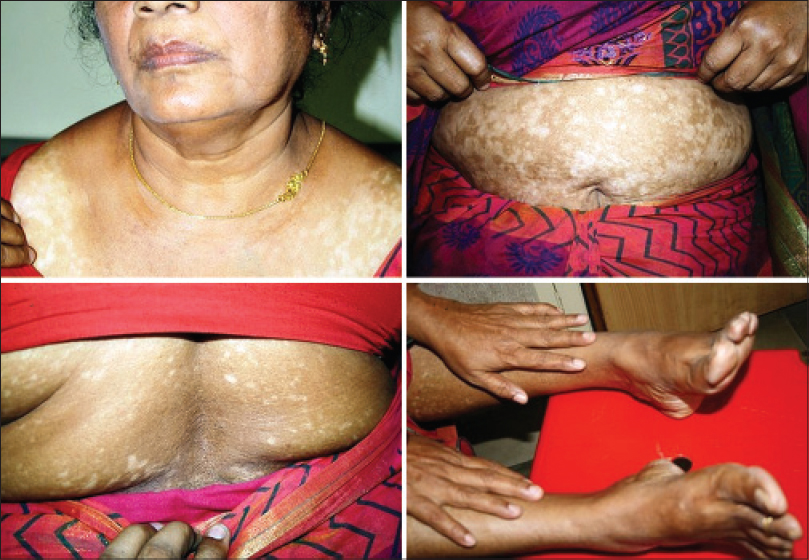
Repetitive segmentations, unilateral or bilateral were recorded in both localized [Figure - 1] and disseminated forms of the disease [Figure - 2]. Linear, curvilinear and circular bands around appendages and orifices on the face were consistent with Blaschko's patterns and all such segmentations on the face are shown as a composite diagram [Table - 1]. When compared across patients, these patterns were found complementary to one another, akin to pieces in a jig-saw puzzle [Figure - 3] and [Figure - 4]. On the rest of the body, larger anatomical segmentations were observed delineating the head and neck, trunk, limbs and their smaller extensions/appendages as shown in the total body map [Table - 1]. Segmentation patterns observed on the trunk included: head and neck as a single large segment separated from the trunk by a sharp line [Figure - 5]a, a broad “V” shaped segment covering the shoulders in the front and the back [Figure - 6], circular bands involving the breasts [Figure - 7] and umbilicus [Figure - 5]a, bilateral vertical bands alongside the spine [Figure - 8] and similar vertical bands between the milk lines in the front [Figure - 5]a, a horizontal line at the level of the umbilicus dividing the trunk into large upper and lower segments [Figure - 2], the latter patterned as a bathing trunk segment formed by the lower trunk joining with the lower limbs on either side [Figure - 9]a and [Figure - 9]b. Fulminate disease on the trunk demonstrated Blaschko's line/band patterns [Figure - 10] which were highlighted in a patient with evolving vitiligo patches interspersed with a preexisting giant melanocytic nevus, both assuming Blaschkoid lines.
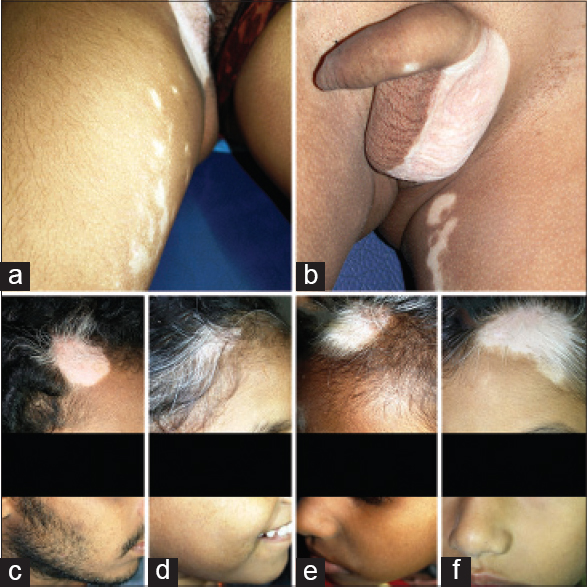 |
| Figure 1: (a and b) Focal lesions with similar linear genital segments involved on either side of midline in both sexes. (c-f) Similar circular segmentations on either side of midline on the scalp margins |
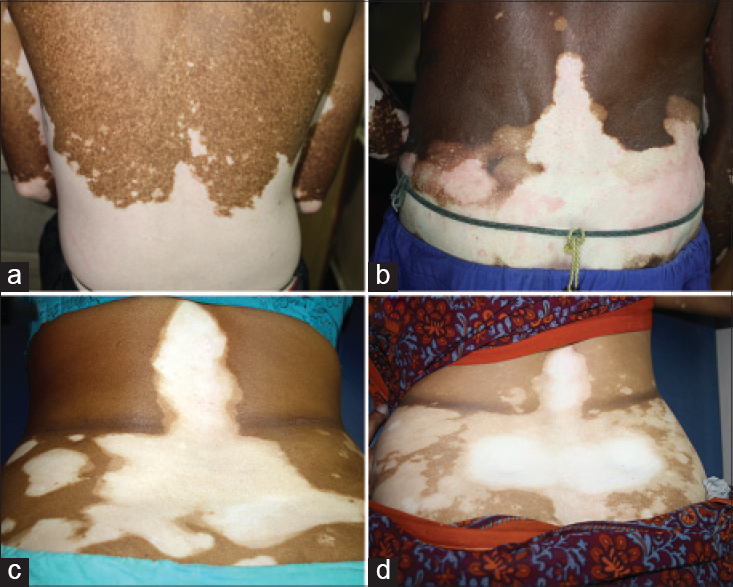 |
| Figure 2: (a-d) Segmentation line across the trunk at the waist line with a vertical band-like extension upward, along the spine |
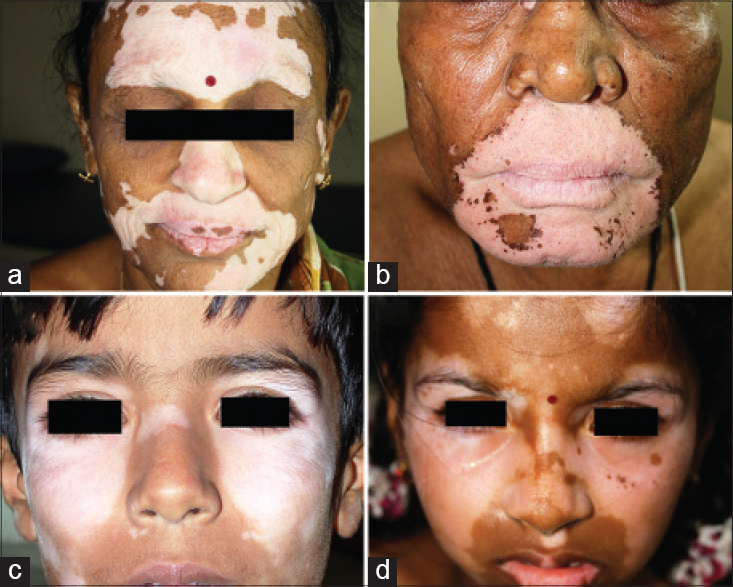 |
| Figure 3: Bilateral segments as jigsaw pieces on the face in different patients: Circular mosaics around the mouth are involved in patients (a and b). The same segment is spared in patients (c and d) |
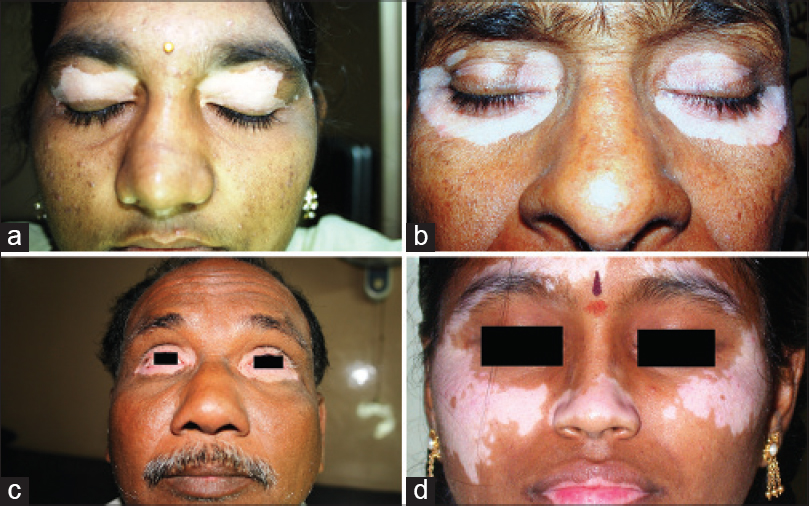 |
| Figure 4: Focal disease with bilateral lesions involving only the face and different orbital segments randomly affected. (a) Mirror image lesions on both the upper lids. (b and c) both upper and lower eye lids involved with leukotrichia. (d) Eye lids totally spared with bilateral outer orbital involvement |
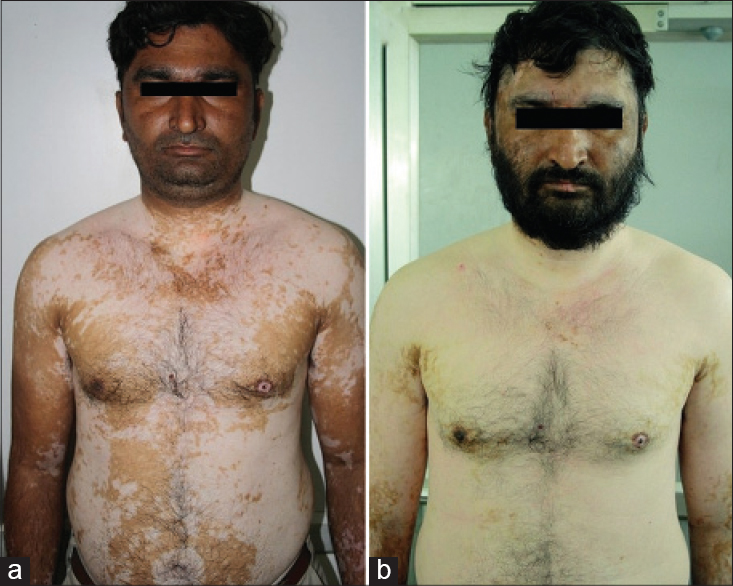 |
| Figure 5: (a) Trunk dominant generalized vitiligo with segmentation lines separating uninvolved head and neck, milk lines with vertical central bands and circular segments around the umbilicus and areola (left). (b) Same patient 2 years later with disease extended to the head and neck. Note: All the earlier segmentation lines on the trunk were obliterated with near universal involvement |
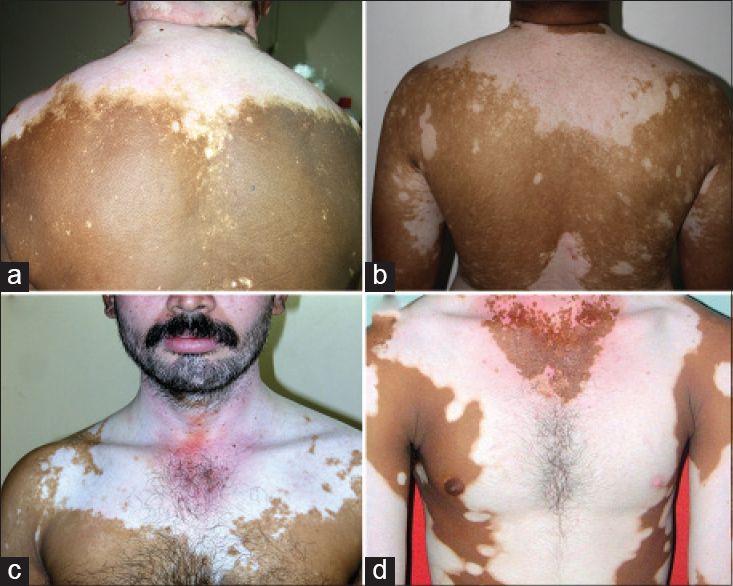 |
| Figure 6: (a-c) Shoulder cloak-like wide “LV” shaped segmentation lines separating the trunk from the upper limbs (d) Mirror image progression on both halves of the trunk with clear shoulder and neck lines. Neck segment shows regression with repigmentation, while the adjacent shoulder and trunk segments remained stable. Note the sparing of the right nipple and areola |
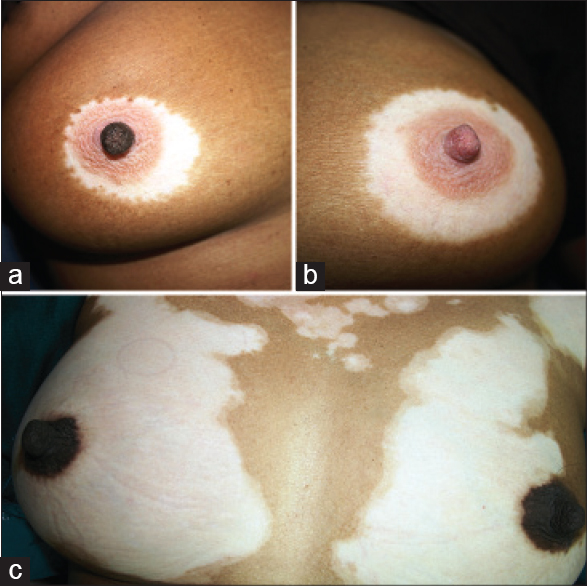 |
| Figure 7: (a and b) Patient with focal vitiligo limited to bilateral involvement of the breasts in three circular segments with the right nipple spared. (c) Progressive generalized vitiligo with large outer circular segmentations of the breasts with the areola and nipple segments spared on both sides |
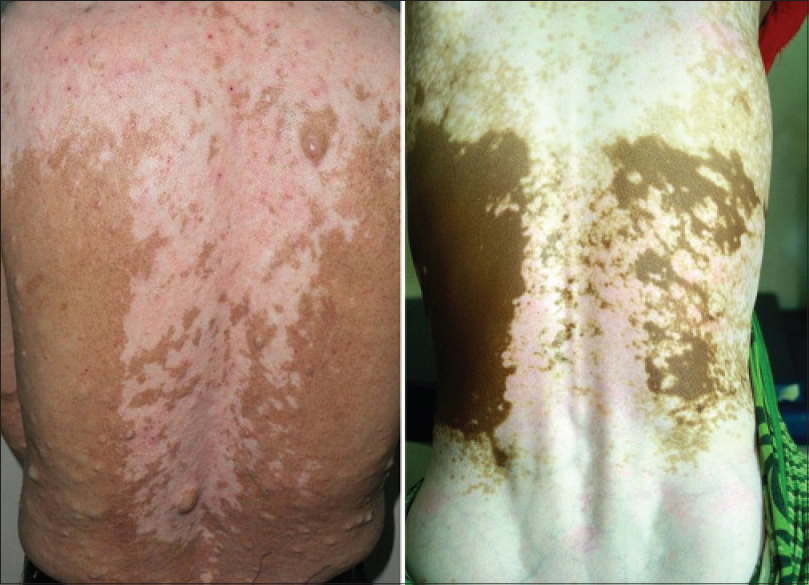 |
| Figure 8: Bilateral vertical bands along the spine which complement the evolving vertical bands illustrated in Figure 2 |
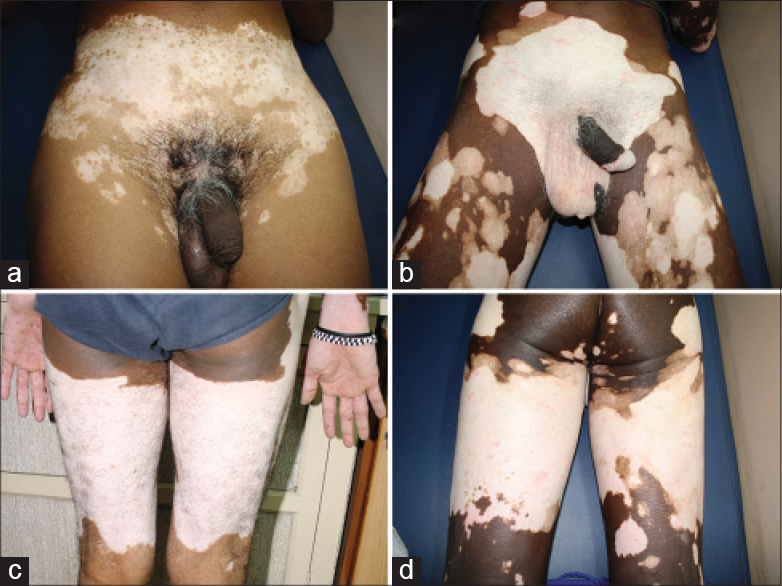 |
| Figure 9: (a and b) Bathing trunk segmentation on the lower trunk. (c and d) Mirror image segmentation on the posterior thigh, from the gluteal fold to the poplitial segment |
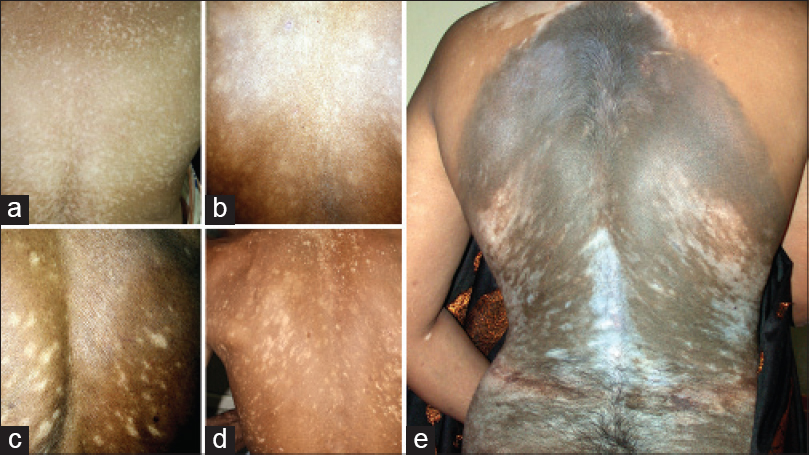 |
| Figure 10: (a-d) Fulminate evolving trunk lesions along Blaschko's lines. (e) Generalized vitiligo associated with large hairy pigmented nevus; both showing Blaschkoid patterns |
On the lower limbs, the thigh, knee, leg and foot segments were highlighted by four lines. The first line was seen across the gluteal and inguinal areas [Figure - 9]a and [Figure - 9]b, second and third lines above and below the knees [Figure - 11] and the fourth delineated the dorsal and plantar aspects of the feet [Figure - 12]. On the upper limbs, prominent wrist lines [Figure - 13] separated the arm and the hand segments. Cubital segments were not as clear as the knee segments observed on the lower limbs.
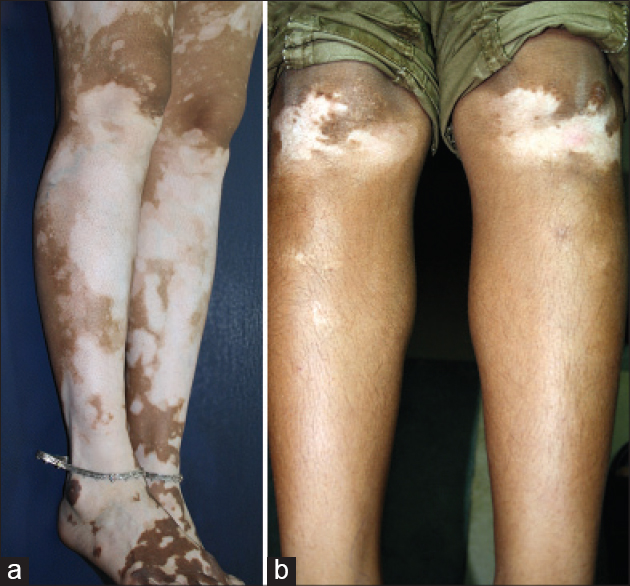 |
| Figure 11: Complimentary segments: (a) Sharp segmentation lines between the knee and legs. (b) Segmentation lines demarcating the knees with the upper line complementing thigh segments shown in [Figure 9]c and [Figure 9]d |
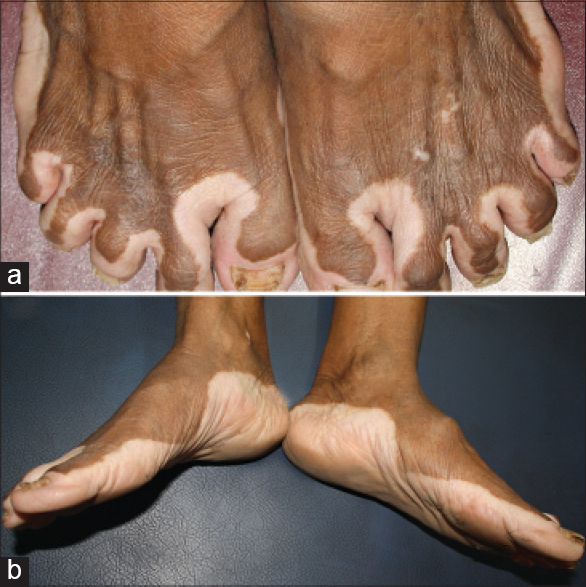 |
| Figure 12: Mirror image segmentations over the feet delineating the dorsal (a) and plantar (b) aspects |
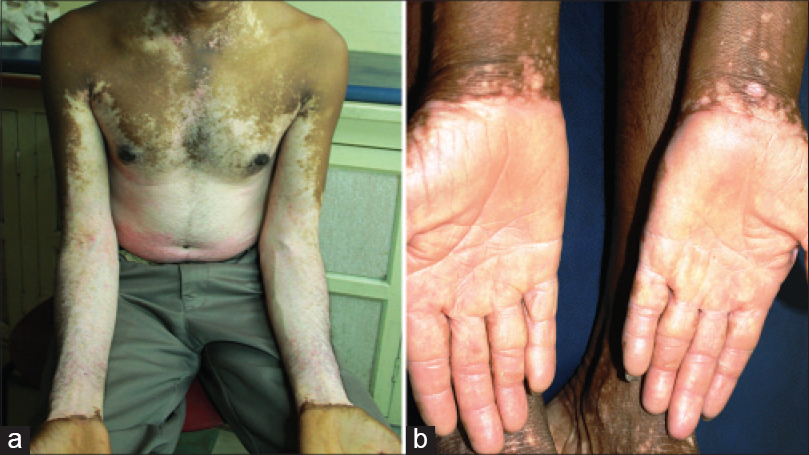 |
| Figure 13: Complementary segments: (a) Generalized vitiligo with sharp demarcation lines at the wrists, with the hands uninvolved. (b) Acral vitiligo sharply limited proximally by the wrist lines |
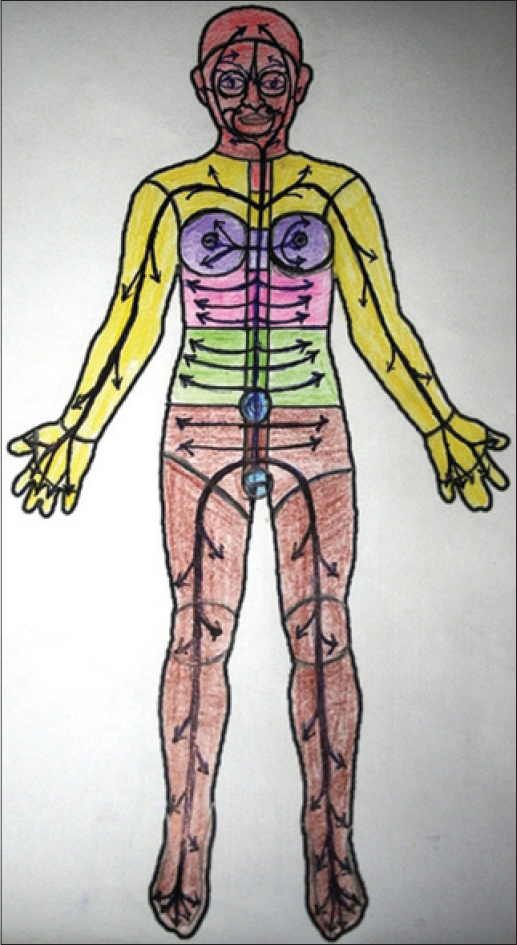
All the recorded segments on the face and the rest of the body were complementary to one another and when assembled together provided a total body map of anatomical divisions [Table - 1]. Unilateral/bilateral and small/large segmentations appeared to be involved at random in individual cases [Figure - 3], [Figure - 4] and [Figure - 9]. Segmentations which were obvious during the relatively stable phases were obliterated when rapid progression extended across adjacent anatomical segments [Figure - 5]a and [Figure - 5]b.
Stable mirror images and perfectly symmetric progressive and regressive patterns were recorded in many instances [Figure - 14], [Figure - 15],[Figure - 16]. Unlike spotted follicular repigmentation seen during the regressive phase in early cases, spontaneous repigmentation in cases of long-standing universal vitiligo progressed in a slow wave-like fashion on the head and neck, in a dorso-ventral direction [Figure - 17] and [Figure - 18] and on the extremities in a proximal to distal centrifugal direction with the acral areas being the last to repigment [Figure - 19].
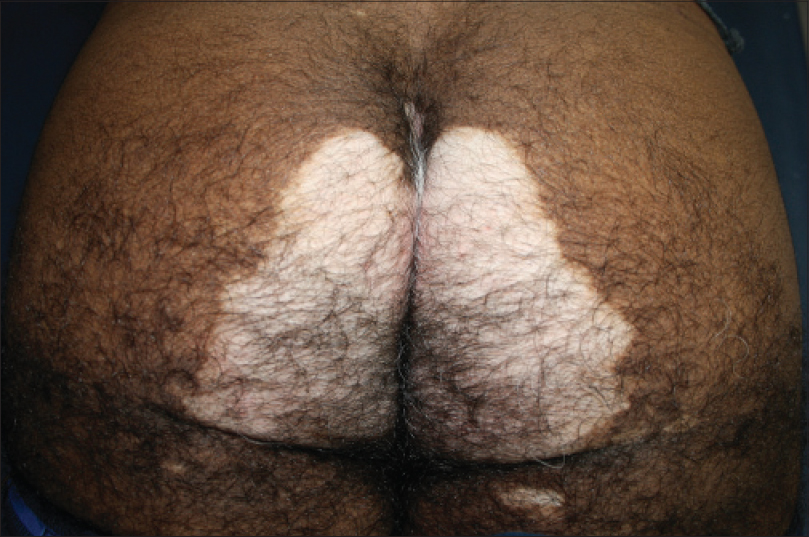 |
| Figure 14: Peri-anal mirror image lesions in multi focal disease |
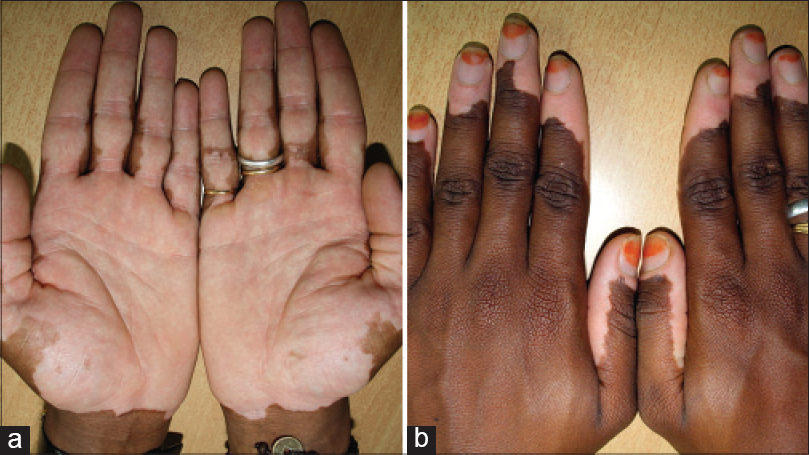 |
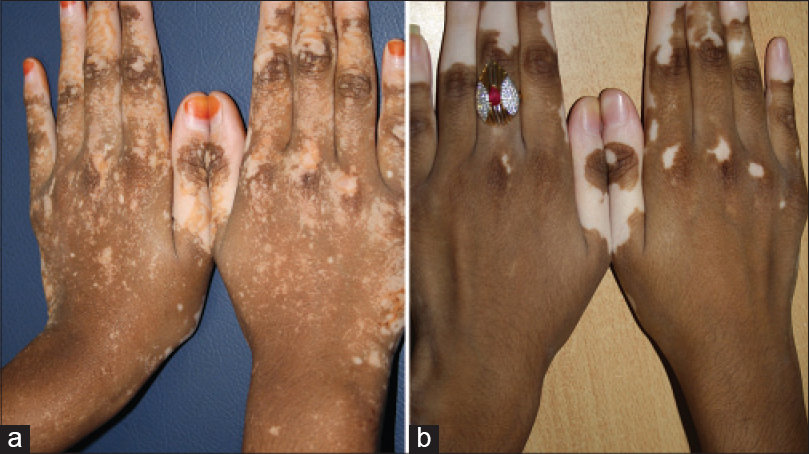
| Figure 15: (a) Mirror image progression on hands stopping short of the wrist lines. (b) Different case from (a) mirror image progression from the tips of fingers towards the wrist lines |
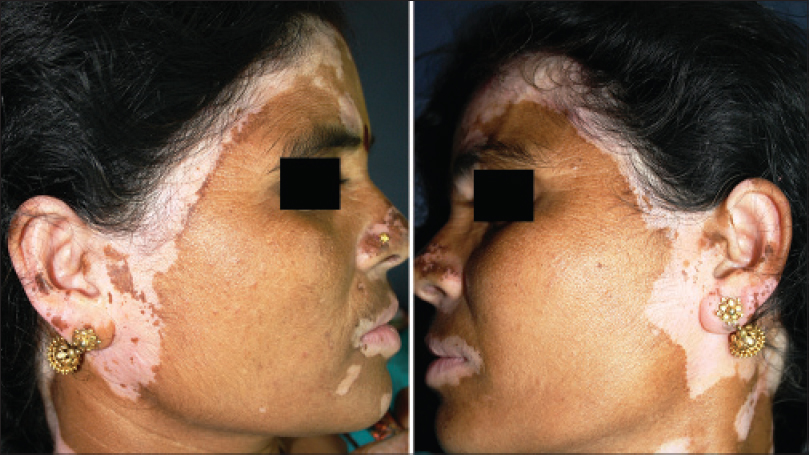 |
| Figure 16: Mirror image bilateral progression from the scalp on to the face |
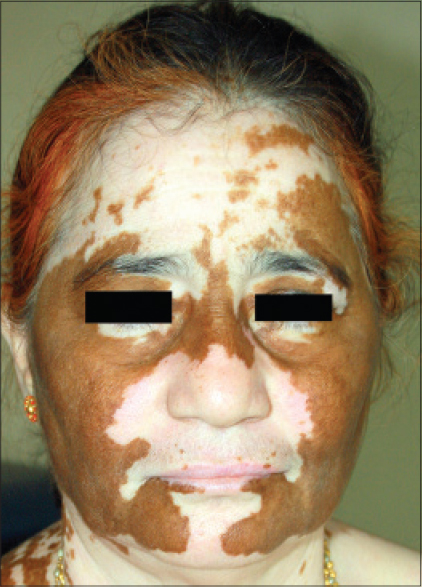 |
| Figure 17: Universal stable vitiligo of 40 years duration showing spontaneous pigment recovery in the recent 6 months- an advancing wave with perfect bilateral symmetry |
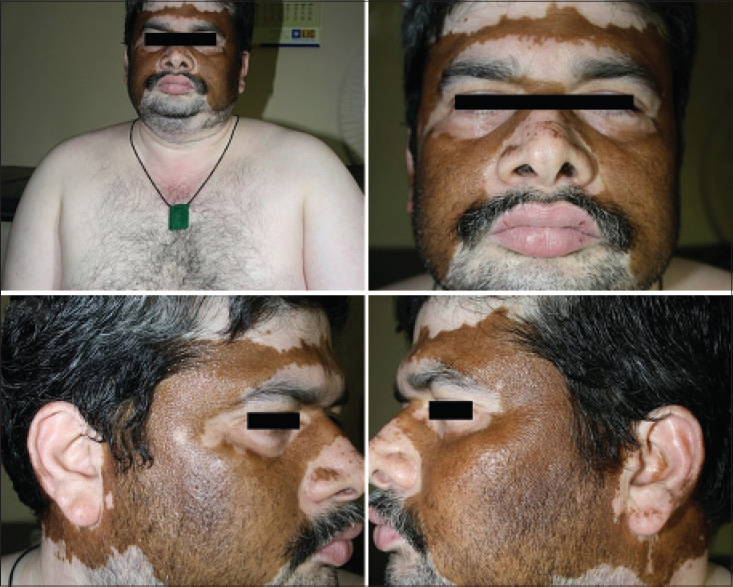 |
| Figure 18: Universal stable vitiligo of several years showing spontaneous symmetric pigment recovery outlining the segmentations of the face |
 |
| Figure 19: (a) Symmetrical progression in generalized vitiligo from the tips of fingers to the wrist line. (b) Same patient after 6 months showing symmetrical regression in the opposite direction |
Discussion
Segmental vitiligo derives its name from the “segmented” appearance of lesions. The other types of vitiligo are differentiated by the absence of such segmentations. Since what actually constitutes a “segment” is not precisely defined, segmental vitiligo is usually identified when lesions present with a sharp mid line restriction or appear along Blaschko's lines/bands or are seen in a quasi-dermatomal distribution. For the first time, this study demonstrated segmentations restricted by horizontal and circular lines across all types of vitiligo, indicating large and small anatomical divisions of the body. How and why such segmentations hitherto escaped notice may have two explanations: most vitiligo studies are cross-sectional due to the lack of long-term follow-up with patients often changing clinicians in the hope of finding quick and early relief and second, segmentations are usually observed only when the disease becomes stable. Evolving lesions which have not yet reached their anatomic (segmental) boundaries would be difficult to recognize. Segmented pattern is considered the hallmark of segmental vitiligo because stability comes early and persists for a long period thus making it easy to identify. However, when segmented lesions are seen bilaterally or in multiple areas, the possibility of disseminated disease presenting with segmentations is not considered, as the current practice is to term that “bilateral or pleuri-segmental vitiligo” or “mixed vitiligo.” It is common knowledge that early evolving segmental vitiligo is also difficult to identify until focal disease extends and stops short at the segmentation lines. For the same reason, segmentations in generalized vitiligo may not have been identified in cross-sectional studies. Moreover, it is difficult to identify segmentations in a progressive disease when several anatomical segments are concurrently involved with lesions spilling over onto contiguous segments [Figure - 5]a and [Figure - 5]b. Identification of segmentations in our study was made easier because involved as well as uninvolved segments in different patients complemented one another in a jigsaw fashion [Figure - 3] and [Figure - 4]. Segmental involvement was observed in different combinations and permutations within the large anatomic segments of the body as well, i.e. head and neck, trunk and upper/lower extremities. Interestingly, some of these major anatomic parts of the body were entirely spared; the trunk in acral vitiligo and the extremities in trunk-dominant generalized vitiligo [Figure - 20].[17] The natural history of vitiligo is spread over many decades with the duration of progressive, stable and regressive phases being unpredictable. Considering that segmentation lines only appear during stable phases or transiently in progressive disease, it was fortuitous that anatomical segmentation lines covering the entire body surface area could be identified in our study covering a short span of 12 years.
 |
| Figure 20: Fulminant evolution of vitiligo (3 months) on the trunk with total sparing of the head and neck and the limbs |
We are not aware of any previous reports of vitiligo lesions conforming to anatomical segmentations as well as Blaschko's lines. The prototype for patterns of cutaneous mosaicism is the system of Blaschko's lines.[18],[19] Lines of Blaschko are invisible under normal conditions and become apparent when the patterns are assumed by many different nevoid and acquired skin diseases. These lines are believed to be a result of mosaicism as they trace the path of embryonic migration. As segmentations in segmental vitiligo do not quite conform to Blaschko's lines, a new “segmental vitiligo pattern” has also been proposed.[20] Nevertheless, somatic cutaneous mosaicism has been suggested in segmental vitiligo.[21],[22],[23],[24] Anatomical segmentations and Blaschko's line patterns observed in all types of vitiligo in this study are in support of this hypothesis. Suspicion of mosaicism arises with visual impressions of “clinical mosaicism” which has a limited connotation. The most compelling evidence for mosaicism in vitiligo is the observation of repetitive and complementary segments in different patients, akin to a jigsaw puzzle. Such a phenomenon we believe cannot be explained by any other hypothesis. However, a biological basis needs to be explored. The human body develops as a conglomerate mosaic of large and small anatomical segments to serve different functions. How an acquired disease relates to such larger segments is a difficult puzzle and explanations could be considered speculative. However, it is common knowledge that mosaicism is a manifestation of developmental defects either genetic or somatic and indeed a strong support for the mosaicism hypothesis in vitiligo comes from defective melanocyte populations (melanocytorrhagy) reported in non-segmental vitiligo.[25],[26],[27],[28],[29]
The genetic and cellular basis of cutaneous mosaicism has been extensively studied in many congenital and acquired skin diseases.[30],[31],[32] Mosaicism is a complex and interesting subject under which segmental vitiligo has been considered, along with other inflammatory polygenic diseases that can also present segmented patterns.[24] Perhaps, most relevant to our observations are the epigenetic cutaneous mosaic manifestations caused by mutations that may affect the structure/function of cellular progeny at different levels of embryonic development. Those affected could be (a) ectodermal stem cells that supply clonal ectodermal units resulting in widespread bilateral Blaschkoid patterns, (b) epidermal stem cells resulting in localized segmented mosaics and (c) single or some epidermal proliferation units at a peripheral level resulting in smaller nevoid aberrations and tumors.[32] This analogy applies to vitiligo which can present as single or multiple segmented lesions in a random selection due to mutational errors along the melanocyte migratory pathway which closely follows the development of skin. Blaschko's lines and anatomical segmentations in vitiligo are not contradictory as the former represent the finer anatomical segmentations of the skin while the latter represents the segmental development of big and small appendages of the body along with that of skin.
A strong argument against the mosaicism hypothesis in vitiligo is that unlike ectodermal nevoid developmental defects which appear at an early age and persist throughout life, vitiligo appears at all ages and has remissions and relapses. The answer may lie in the embryonic development of melanocytes which are not of ectodermal origin. Melanocyte precursors migrate from the neural crest in a long path between the ectodermal and mesodermal layers dorso-ventrally and pass through numerous lateral channels to reach the basal layer of the skin.[33],[34] Mutations that may occur at different stages of such migration [Figure - 21] could explain vitiligo presenting as focal, multifocal or generalized disease with bilateral symmetry. The migratory flow necessarily undergoes stopovers and directional changes at the anatomical junctional areas. Mutations that may occur at such vulnerable points limit the defective progeny to one or all the distal anatomical segments. Anatomical segments in vitiligo may harbor developmentally compromised melanocyte populations with anchoring/functional defects. Their early/easy destruction could be triggered by many external (friction/scratching, contact with phenolic chemicals, sun burn etc.) or internal provocations (oxidative stress, autocytotoxicity, autoimmunity etc.) or combinations of both as epiphenomena. This is consistent with the opinion, “unknown genetic predisposing factors may affect first the skin pigmentary system and the secondary activation of the skin immune/inflammatory responses lead to the expression of the disease.” [35] Unlike ectodermal mosaic defects, the natural history of vitiligo is influenced by an active pigment homeostatic mechanism that can counter and replace destroyed melanocytes. We believe this aspect has relevance to mirror images, symmetrical progressions and regressions seen as unique features in bilateral disease.
 |
| Figure 21:Graphic presentation of anatomical junctions and presumed melanocyte migratory pathways that may persist in adult life |
Pigment homeostasis in vitiligo
Melanocyte stem cells reported to persist in the follicular bulge are believed to initiate perifollicular repigmentation during the regressive phase of vitiligo.[36] However, we have reported histological evidence of persisting micro-inflammation in long standing lesions of vitiligo resulting in permanent follicular loss thus disabling local mechanisms of melanocyte recovery.[16],[37] Common lineage cells such as Schwann cells transforming into melanocytes has been reported in vitro, in chick embryo studies.[34] However, when the disease becomes generalized and extensive, local mechanisms may not be adequate and fresh migration of the central stem cell pools might need to be reactivated to counter the pigment loss.
Progressive, regressive and stable phases concurrently seen in different anatomical segments are a common observation. It is probable that each anatomical segment may have its own regional melanocyte stem cell niches distinct from the follicular bulge stem cells. Therefore, these anatomical segments may function as independent pigment homeostatic units and when affected at different periods can present different phases of the disease. Although stem cell niches other than those in the hair follicle have not been reported, it is interesting that in very long-standing cases, spontaneous pigment recovery was observed in bilateral symmetric progressive patterns [Figure - 17] and [Figure - 18]. This raises the possibility of renewed melanocyte migration from stem cell populations that may persist at strategic anatomical junctions along the original pathway from the neural crest. Local (follicular), regional and central stem cell aggregations may serve as stepwise defense mechanisms for pigment homeostasis against progressive autoimmune destruction of melanocytes. It is difficult to speculate whether autoimmune destruction affects only the melanocytorrhagic mosaics or extends to the surrounding normal mosaics simply as a cascading effect or epitope spreading. The presumption that vitiligo affects only melanocytorrhagic segments can be tested in stable mono-segmental vitiligo cases by comparing melanocytes cultured from the affected segment and distant uninvolved skin.
Remissions and relapses are hallmarks of autoimmune diseases. Cessation of autoimmune activity on bilateral fronts simultaneously can explain the mirror images observed in vitiligo. Bilateral progression/regression appears to be an outcome of the two systemically opposing forces, autoimmune destruction and pigment homeostasis, the latter being a reparative mechanism, pigment recovery seen as a much belated response. Lack of long-term studies has been a huge lacuna in assessing the natural history of vitiligo. For the first time, we are reporting patterned spontaneous regression in some long-standing cases of vitiligo which we believe is due to belated pigment homeostasis. However, as these numbers are small, larger follow-up studies, especially involving cases of near-universal vitiligo, are required to throw greater light on this interesting phenomenon.
Acral versus generalized vitiligo
Progression patterns of acral vitiligo [Figure - 12] and [Figure - 15] and generalized vitiligo with trunk dominance [Figure - 20] appear to be different. Acral vitiligo is the most common among all types in our population with bilateral lesions initially appearing on the most distal areas (last in the embryological development sequence). Trunk involvement is uncommon and when it occurs, is a late phenomenon.[17] It is tempting to speculate that areas that are distant from the neural crest (i.e., acral areas) become more vulnerable to mutations because of the longer migratory paths with an increasingly greater number of anatomical junctions to be negotiated by the melanocyte precursors. Acral areas are also devoid of mature hair follicles. When the local stem cell reservoirs are depleted, melanocytes can only be replaced by renewed migration from the regional or central stem cell pools, thus delaying pigment recovery. This factor might also explain the intransigence of acral lesions to treatment.
Implications of segmentations in vitiligo
Dichotomy of vitiligo as segmental/non-segmental types becomes untenable when vitiligo is perceived as mono-segmented or multi-segmented disease. A simple understanding of vitiligo as focal or generalized disease with multifocal forms bridging the two has a definite prognostic value similar to that observed in alopecia areata, another micro-inflammatory disease in which autoimmune mechanisms are also implicated. Good results obtained in mono-segmented vitiligo with melanocyte grafts from uninvolved areas in the same patient supports the hypothesis that vitiligo affects mosaics populated by inherently defective melanocytes.[38] Controlling autoimmunity in the progressive generalized form can be a difficult task requiring stronger measures such as systemic steroids, akin to the treatment of other autoimmune skin diseases. However, vitiligo is not a life-threatening problem and their usage needs to be balanced against possible side effects. Once the disease progression is arrested, natural pigment homeostatic mechanisms are likely to repair the damage in due course. However, battling with sustained/recurring autoimmune mechanisms in generalized disease may not yield satisfactory results. Patient compliance can be improved with proper counseling regarding the autoimmune nature of the disease and the inevitably long process of pigment recovery. Currently, phototherapy is the only mechanism by which follicular stem cells can be stimulated. Future research to find additional means to activate central regulatory mechanisms involved in pigment homeostasis may open up new options for treatment.
Conclusions
Similar unilateral/bilateral segmented patterns delineating major/minor anatomical divisions of the body may indicate a preexisting developmental defect such as mosaicism. The specific nature of melanocytorrhagy and its applicability as the biological mosaic defect needs to be further investigated.
Limitations
A retrospective study can only reveal certain observations which might have escaped attention in routine examination. The observations derived should be confirmed by prospective studies.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
| 1. |
Bystryn J. Theories on the pathogenesis of depigmentation: Immune hypothesis. In: Hann KS, Nordlund JJ, editors. Vitiligo: A Monograph on the Basic and Clinical Science. 1st ed. Oxford, London: Blackwell Science Ltd.; 2000.p. 129-37.
[Google Scholar]
|
| 2. |
Njoo MD, Westerhof W. Vitiligo. Pathogenesis and treatment. Am J Clin Dermatol 2001;2:167-81.
[Google Scholar]
|
| 3. |
Le Poole IC, van den Wijngaard RM, Westerhof W, Das PK. Presence of T cells and macrophages in inflammatory vitiligo skin parallels melanocyte disappearance. Am J Pathol 1996;148:1219-28.
[Google Scholar]
|
| 4. |
Le Poole IC, Wañkowicz-Kaliñska A, van den Wijngaard RM, Nickoloff BJ, Das PK. Autoimmune aspects of depigmentation in vitiligo. J Investig Dermatol Symp Proc 2004;9:68-72.
[Google Scholar]
|
| 5. |
Le Poole IC, Das PK, van den Wijngaard RM, Bos JD, Westerhof W. Review of the etiopathomechanism of vitiligo: A convergence theory. Exp Dermatol 1993;2:145-53.
[Google Scholar]
|
| 6. |
Lotti T, Hautmann G, Hercogova J, editors. Vitiligo: Disease or symptom? From the confusion of the past to current doubts. In: Vitiligo. Problems and Solutions. New York: Marcel Dekker Inc.; 2004. p. 1-15.
[Google Scholar]
|
| 7. |
Ezzedine K, Lim HW, Suzuki T, Katayama I, Hamzavi I, Lan CC, et al. Revised classification/nomenclature of vitiligo and related issues: The vitiligo global issues consensus conference. Pigment Cell Melanoma Res 2012;25:E1-13.
[Google Scholar]
|
| 8. |
Nordlund JJ. Vitiligo: A review of some facts lesser known about depigmentation. Indian J Dermatol 2011;56:180-9.
[Google Scholar]
|
| 9. |
Hann SK, Sungbin I. Clinical variants of vitiligo. In: Lotti T, Hercogova J, editors. Vitiligo – Problems and Solutions. NewYork: Marcel Dekker; 2004.p. 159-73.
[Google Scholar]
|
| 10. |
Hann SK, Lee HJ. Segmental vitiligo: Clinical findings in 208 patients. J Am Acad Dermatol 1996;35 (5 Pt 1):671-4.
[Google Scholar]
|
| 11. |
Khaitan BK, Kathuria S, Ramam M. A descriptive study to characterize segmental vitiligo. Indian J Dermatol Venereol Leprol 2012;78:715-21.
[Google Scholar]
|
| 12. |
van Geel N, De Lille S, Vandenhaute S, Gauthier Y, Mollet I, Brochez L, et al. Different phenotypes of segmental vitiligo based on a clinical observational study. J Eur Acad Dermatol Venereol 2011;25:673-8.
[Google Scholar]
|
| 13. |
Taieb A, Picardo M, editors. Epidemiology, definitions and classification. In: Vitiligo.Berlin: Springer-Verlag; 2010.p. 13-23.
[Google Scholar]
|
| 14. |
Ezzedine K, Gauthier Y, Léauté-LabrÜze C, Marquez S, Bouchtnei S, Jouary T, et al. Segmental vitiligo associated with generalized vitiligo (mixed vitiligo): A retrospective case series of 19 patients. J Am Acad Dermatol 2011;65:965-71.
[Google Scholar]
|
| 15. |
Mulekar SV, Al Issa A, Asaad M, Ghwish B, Al Eisa A. Mixed vitiligo. J Cutan Med Surg 2006;10:104-7.
[Google Scholar]
|
| 16. |
Attili VR, Attili SK. Segmental and generalized vitiligo: Both forms demonstrate inflammatory histopathological features and clinical mosaicism. Indian J Dermatol 2013;58:433-8.
[Google Scholar]
|
| 17. |
Attili VR, Attili SK. Acral vitiligo and lichen sclerosus – Association or a distinct pattern?: A clinical and histopathological review of 15 cases. Indian J Dermatol 2015;60:519.
[Google Scholar]
|
| 18. |
Molho-Pessach V, Schaffer JV. Blaschko lines and other patterns of cutaneous mosaicism. Clin Dermatol 2011;29:205-25.
[Google Scholar]
|
| 19. |
Happle R. Dohi memorial lecture. New aspects of cutaneous mosaicism. J Dermatol 2002;29:681-92.
[Google Scholar]
|
| 20. |
van Geel N, Speeckaert R, Melsens E, Toelle SP, Speeckaert M, De Schepper S, et al. The distribution pattern of segmental vitiligo: Clues for somatic mosaicism. Br J Dermatol 2013;168:56-64.
[Google Scholar]
|
| 21. |
TaÏeb A, Morice-Picard F, Jouary T, Ezzedine K, Cario-André M, Gauthier Y. Segmental vitiligo as the possible expression of cutaneous somatic mosaicism: Implications for common non-segmental vitiligo. Pigment Cell Melanoma Res 2008;21:646-52.
[Google Scholar]
|
| 22. |
Taieb A. The concept of mosaicism applied toSV. In: Picardo M, Taieb A, editors. Vitiligo. Berlin: Springer-Verlag; 2010. p. 298-301.
[Google Scholar]
|
| 23. |
van Geel N, Mollet I, Brochez L, Dutré M, De Schepper S, Verhaeghe E, et al. New insights in segmental vitiligo: Case report and review of theories. Br J Dermatol 2012;166:240-6.
[Google Scholar]
|
| 24. |
Kouzak SS, Mendes MS, Costa IM. Cutaneous mosaicisms: Concepts, patterns and classifications. An Bras Dermatol 2013;88:507-17.
[Google Scholar]
|
| 25. |
Cario-André M, Pain C, Gauthier Y, TaÏeb A. The melanocytorrhagic hypothesis of vitiligo tested on pigmented, stressed, reconstructed epidermis. Pigment Cell Res 2007;20:385-93.
[Google Scholar]
|
| 26. |
Namazi MR. Neurogenic dysregulation, oxidative stress, autoimmunity, and melanocytorrhagy in vitiligo: Can they be interconnected? Pigment Cell Res 2007;20:360-3.
[Google Scholar]
|
| 27. |
Gauthier Y, Cario Andre M, TaÏeb A. A critical appraisal of vitiligo etiologic theories. Is melanocyte loss a melanocytorrhagy? Pigment Cell Res 2003;16:322-32.
[Google Scholar]
|
| 28. |
Kumar R, Parsad D. Melanocytorrhagy and apoptosis in vitiligo: Connecting jigsaw pieces. Indian J Dermatol Venereol Leprol 2012;78:19-23.
[Google Scholar]
|
| 29. |
Kumar R, Parsad D, Kanwar AJ. Role of apoptosis and melanocytorrhagy: A comparative study of melanocyte adhesion in stable and unstable vitiligo. Br J Dermatol 2011;164:187-91.
[Google Scholar]
|
| 30. |
Chuong CM, Dhouailly D, Gilmore S, Forest L, Shelley WB, Stenn KS, et al. What is the biological basis of pattern formation of skin lesions? Exp Dermatol 2006;15:547-64.
[Google Scholar]
|
| 31. |
Paller AS. Piecing together the puzzle of cutaneous mosaicism. J Clin Invest 2004;114:1407-9.
[Google Scholar]
|
| 32. |
Itin P, Burger B. Mosaic manifestations of monogenic skin diseases. J Dtsch Dermatol Ges 2009;7:744-48.
[Google Scholar]
|
| 33. |
Dupin E, Le Douarin NM. Development of melanocyte precursors from the vertebrate neural crest. Oncogene 2003;22:3016-23.
[Google Scholar]
|
| 34. |
Osawa, M. Melanocyte stem cells [Internet], StemBook, ed. The Stem Cell Research Community; 2009 Jun 30. 10.3824/stembook. 1.46.1, Available from: http://www.stembook.org.
[Google Scholar]
|
| 35. |
Picardo M, Taieb A The somatic mosaicism hypothesis for SV and deductions for NSV– Editors synthesis. In: Picardo M, Taieb A, editors. Vitiligo. Berlin: Springer-Verlag; 2010. p. 314.
[Google Scholar]
|
| 36. |
Falabella R. Vitiligo and the melanocyte reservoir. Indian J Dermatol 2009;54:313-8.
[Google Scholar]
|
| 37. |
Attili VR, Attili SK. Lichenoid inflammation in vitiligo – a clinical and histopathologic review of 210 cases. Int J Dermatol 2008;47:663-9.
[Google Scholar]
|
| 38. |
Olsson M. Surgical therapies-selection of patients. In: Picardo M, Taieb A, editors. Vitiligo. Berlin: Springer-Verlag; 2010. p. 395-407.
[Google Scholar]
|
Fulltext Views
10,604
PDF downloads
3,644





