Translate this page into:
Assessment of liver and renal functions in human immunodeficiency virus-infected persons on highly active antiretroviral therapy: A mixed cohort study
Correspondence Address:
Vikram K Mahajan
Department of Dermatology, Venereology and Leprosy, Dr. R. P. Govt. Medical College, Kangra (Tanda) - 176 001, Himachal Pradesh
India
| How to cite this article: Mahajan VK, Wadhwa D, Sharma A, Chauhan S, Vashist S, Kumar P, Chowdhry B. Assessment of liver and renal functions in human immunodeficiency virus-infected persons on highly active antiretroviral therapy: A mixed cohort study. Indian J Dermatol Venereol Leprol 2020;86:499-507 |
Abstract
Background: Indian data on potential hepatorenal toxic effects of highly active antiretroviral therapy (HAART) in HIV/AIDS-affected persons is lacking.
Objectives: To assess hepatorenal abnormalities in HIV-infected persons on HAART in a hospital-based mixed cohort study using concurrent and nonconcurrent data analysis.
Methods: Hepatorenal function tests, urinalysis and ultrasonogaphy for liver/kidneys (when applicable) were assessed in 400 (men 185; women 215) persons aged 2–84 (mean 47.8) years on HAART. Acute liver toxicity, acute kidney injury and chronic kidney disease were defined depending upon abnormal serum alanine aminotransferase, urea and creatinine levels/clearance as per standard guidelines.
Results: The duration of HAART was 1 month to 9 years (mean 3.7 years) with 284 (71%) individuals being on treatment for ≤5years. The major HAART regimens included zidovudine + lamivudine + nevirapine in 175 (43.8%), tenofovir + lamivudine + efavirenz in 174 (43.5%) and zidovudine + lamivudine + efavirenz in 20 (5%) individuals and were associated with grade-1 hepatic dysfunction in 57 (14.3%) individuals, with men aged between 31 and 45 years on antiretroviral therapy for >5 years being mainly affected. Forty two (17.1%) of 246 individuals with anemia and 15 (9.7%) of 154 individuals without anemia showed hepatic dysfunction. None had acute kidney injury, chronic kidney disease or abnormal urinalysis or ultrasonography. In contrast, the pretreatment elevated serum alanine amiotranerase in 99 (22.3%) and blood urea and/or creatinine levels in 16 (4%) individuals decreased significantly post highly active antiretroviral therapy.
Conclusions: The study reflects the low frequency of regimen based highly active antiretroviral therapy-associated hepatic or nephrotoxicity despite prolonged use, especially in the absence of other risk factors. Preexisting anemia appears an important risk factor for highly active antiretroviral therapy-induced hepatotoxicity (OR 1.90, Cl 95% CI 1.02–3.57, P = 0.04). Highly active antiretroviral therapy-associated nephrotoxicity was not a significant problem. Study of viral load or other risk factors and potential of each drug for hepatorenal toxicity/dysfunction in HIV affected were not part of the study. A small number of subjects and retrospective analysis of biochemical parameters were other important limitations.
Introduction
Human immunodeficiency virus (HIV) infection/acquired immunodeficiency syndrome (AIDS) is a major public health problem worldwide, particularly in developing nations of South-East Asia and Africa. As per World Health Organization (WHO) 2015 estimates, 36.7 million people are living with HIV worldwide despite impressive decline in new cases following HAART. India, with 21.17 lakh persons living with HIV/AIDS, accounts for the third-largest HIV affected in the world with estimated adult (15–49 years) prevalence of 0.26% (0.22%–0.32%) in 2015.[1] Manipur, Mizoram, Nagaland, Andhra Pradesh, Karnataka, Maharashtra, Tamil Nadu, Gujarat and Goa with a prevalence of 0.2% to 1.2% share the major burden.[2] Himachal Pradesh with approximately 70-lakh population had 5,723 HIV/AIDS-affected persons in 2015 with an estimated adult prevalence of 0.1%.[2] Approximately15.8 million people have access to HAART worldwide and the treatment coverage is likely to increase with the current strategy of initiating HAART in all HIV-positive individuals irrespective of CD4 counts.[3] However, potential toxic effects, occasionally life-threatening, of HAART over prolonged periods on hepatocytes and nephrons remain an important limitation for its long-term use.[4] The overall incidence of HAART-related hepatotoxicity varies across studies. It increases the risk of hepatitis C virus co-infection and resultant liver damage.[5],[6] Acute, occasionally fatal, liver necrosis can occur from nevirapine and efavirenz-induced drug hypersensitivity syndrome.[7] Treatment with nucleoside reverse transcriptase inhibitors too has been associated with an overall rate of 12% severe hepatotoxicity.[8] Tenofovir disoproxil fumarate, in particular, gets accumulated actively in proximal renal tubules causing mitochondrial injury and functional disturbance.[9] Although Himachal Pradesh has a low disease prevalence, data on potential hepatorenal toxic effects of HAART in HIV/AIDS-affected persons is lacking. The study was performed with an objective to assess liver and renal functions in HIV-infected persons on HAART. This will help in the provisioning of comprehensive health care for affected persons envisaged in National AIDS Control Program.
Methods
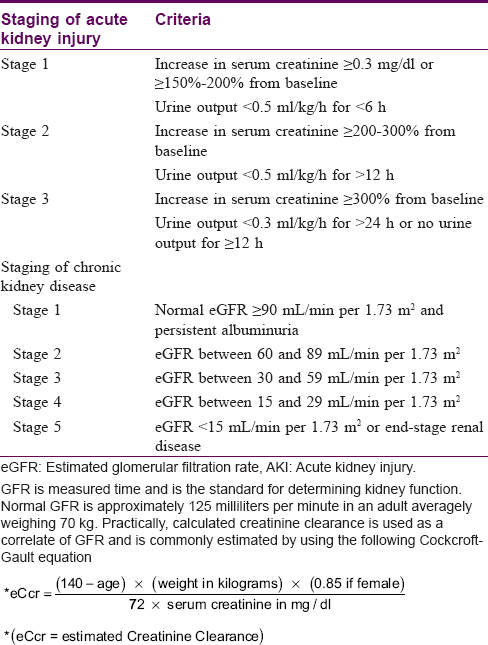
The study design was a combined prospective and retrospective (mixed) cohort study with baseline data recorded at the time of first presentation in the institute affiliated antiretroviral therapy center, and new data (concurrent and nonconcurrent) obtained during the 9-month-study period between Feb and Oct 2017. It comprised 402 persons living with HIV/AIDS consecutively attending antiretroviral therapy center for consultation/drug supply on any day of the week during the study period. Sociodemographic details, HAART regimens with duration, last recorded CD4 counts and liver and kidney function test reports before initiating HAART were noted from antiretroviral therapy-booklets of enrolled subjects. They were examined for any clinical evidence of hepatorenal abnormality which could be attributable to HAART. Liver and kidney function tests and urinalysis, particularly for albuminuria and casts, were repeated in patients having no updated records or when available reports were >1 month old. Abdominal ultrasonography was performed for any hepatorenal abnormality in persons with altered serum alanine aminotransferase, blood urea and serum creatinine levels. Hepatotoxicity was graded based on serum alanine aminotransferase elevations >40.0 international units (IU; upper limit of normal range 6–40.0 IU) based on the AIDS Clinical Trial Group grading [Table - 1].[10],[11] Renal dysfunction was classified as acute kidney injury or chronic kidney disease [Table - 2].[12],[13],[14],[15],[16],[17] Acute kidney injury was defined as the development of any one of the following limits within 48 h: absolute increase in serum creatinine ≥0.3 mg/dl, percentage increase in serum creatinine ≥50% from baseline or decrease in urine output to <0.5 ml/kg/h for >6 h, and its clinical staging was according to diagnostic criteria by Acute Kidney Injury-Network based on serum creatinine.[12] Chronic kidney disease was defined and classified as kidney damage manifesting with albuminuria or decreased renal functions quantified by estimated glomerular filtration rate (eGFR) persisting for more than 3 months.[13],[14],[15] Staging of chronic kidney disease severity was as per National Kidney Foundation–Kidney Disease Outcomes Quality Initiative Classification.[14],[16],[17]

Data analysis
The recorded data for two patients was incomplete and they were excluded from final analysis. Mann–Whitney nonparametric test was used for variables that were not distributed normally. The χ[2] test/Student's t-test and one-way ANOVA test were used for statistical analysis of the categorical and parametric data. A P value <0.05 calculated at 5% level (95% confidence interval) was considered statistically significant.
Results
The baseline characteristics of study subjects are shown in [Table - 3]. There were 185 (46.3%) males and 215 (53.8%) females aged between 2 and 84 (mean 47.8) years with majority, 302 (75.5%) individuals, being aged 16–45 years. None of them had other comorbidities like diabetes or hypertension. The majority, 223 (55.8%) individuals had CD4 counts of 200–500 cells/microliter and were in WHO stage-1 of the HIV disease while counts were <200 cells/microliter in 60 (15%) individuals. All were on regular highly active antiretroviral therapy for 1 month to 9 years (mean 3.7 years) and the majority, 284 (71%) individuals, were taking treatment for ≤5 years. The major antiretroviral therapy regimens comprised zidovudine + lamivudine + nevirapine in 175 (43.8%) and tenofovir + lamivudine + efavirenz in 174 (43.5%) individuals. The initial nevirapine-based antiretroviral therapy regimens in 6 (1.5%) persons were changed to tenofovir + lamivudine + efavirenz due to nevirapine hypersensitivity. Twenty nine (7.3%) individuals had been treated for pulmonary tuberculosis before HAART initiation. The only HBV co-infected subject had not received any additional drugs.

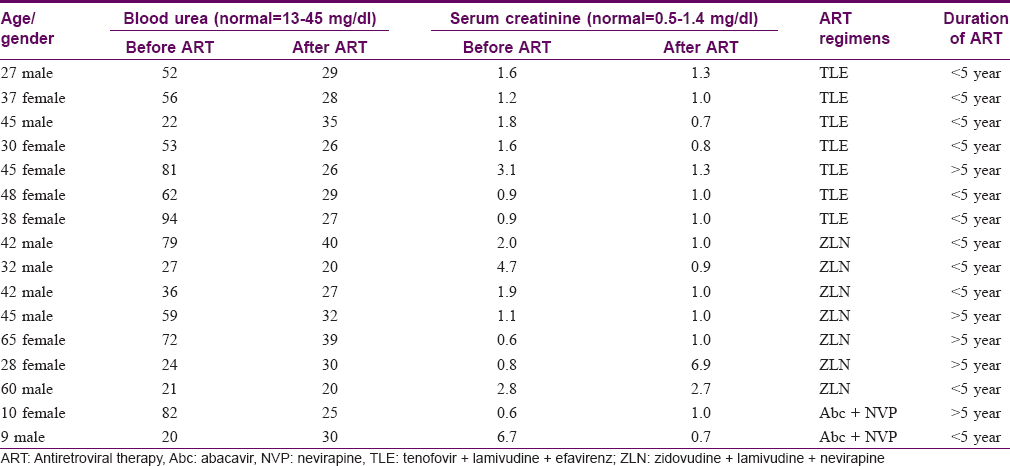
[Table - 1] and [Table - 4] depict mean values of biochemical parameters with antiretroviral therapy duration. The mean values of serum alanine amiotransferase levels decreased significantly after antiretroviral therapy in 99 (22.3%) individuals; 83 with grade-1, 15 with grade-2 and one with grade-3 hepatic dysfunction, particularly among individuals on tenofovir + lamivudine + efavirenz and abacavir + nevirapine regimens. The mean values of serum alanine aminotransferase before highly active antiretroviral therapy were 39.7 ± 26.4 IU which decreased to 32.1 ± 17.0 and 31.0 ± 14.0 IU in patients with ≤5 years and more than 5 years of HAART respectively. The difference was statistically significant suggesting improvement after therapy. Further analysis of hepatic dysfunction revealed that 57 (14.3%) individuals with normal serum alanine aminotransferase levels before HAART developed grade-1 hepatic dysfunction after therapy [Table - 1] and [Table - 5]. Among them, 36 (63.2%) individuals were aged between 31 and 45 years, 26 (45.6%) were on antiretroviral therapy for >5 years, and men outnumbered women by one and half times [Table - 3]. However, the same antiretroviral therapy regimen was continued in all these patients by the treating physician.


Anemia (hemoglobin ≤11 g/dl) was present in 246 (61.5%) persons. Amongst this population, 42 (17.1%) versus 15 (9.7%) of the 154 individuals without anemia showed hepatic dysfunction suggesting HIV-affected persons with preexisting anemia are at increased risk for HAART-induced hepatotoxicity (OR 1.90, Cl 95% CI 1.02–3.57, P = 0.04).
None of the HIV-affected individuals fulfilled acute kidney injury or chronic kidney disease criteria before or after HAART or showed any investigative abnormality. Before HAART, the baseline values (mean±SD) for blood urea and creatinine were 28.4 ± 10.4mg/dl and 0.9 ± 0.3 mg/dl, respectively. These baseline values did not show significant alteration in patients who had received HAART either for ≤5 years or >5 years [Table - 1]. The increased blood urea levels (>40 mg/dl) in ten patients and elevated serum creatinine (>1.4 mg/dl) levels in eight individuals, decreased after antiretroviral therapy most significantly in individuals on tenofovir + lamivudine + efavirenz, tenofovir + lamivudine + nevirapine or abacavir + nevirapine regimens [Table - 4] and [Table - 6].
There was history of nevirapine hypersensitivity in six, and treatment for pulmonary tuberculosis prior to initiating HAART in 29 individuals but no abnormality in hepatorenal function tests was noted. There was no information on alcohol abuse or concurrent infections, their treatment or prophylaxis, especially for Pneumocystis jiroveci pneumonia or viral hepatitis, recorded in the antiretroviral therapy booklet.
Discussion
The overall demographic profile of our study subjects was similar to that described in our previous report.[18] The majority, 75.5% persons, were between 16–45 years of age and women outnumbered men by 1.7 times. All individuals were on regular antiretroviral therapy for 1 month to 9 years (mean 3.7 years) having mean CD4 counts of 408.9 (range 33–1254) cells/μl. The majority, 223 (55.8%) individuals, had CD4 counts of 200–500 cells/μl and were in WHO stage-1 of the HIV disease.
All antiretroviral therapy drugs have been reported to cause hepatotoxicity varying from transaminitis to frank liver failure and nephrotoxicity varying from acute kidney injury to chronic kidney disease.[19],[20],[21],[22] Further, highly active antiretroviral therapy-induced hepatotoxicity with a prevalence of 12% to 23% remains one of the major concerns for treatment compliance and adherence, and possibly drug resistance.[8],[23] Nucleoside reverse transcriptase inhibitors (nevirapine, efavirenz, etravirine), in particular, have been associated with severe hepatotoxicity with overall prevalence of 12 per cent.[8] Nevirapine and tipranavir (a protease inhibitor) are particularly notorious in causing severe liver failure. Tipranavir is reported to cause grade-3 alanine aminotransferase elevations in 6.3% of patients.[24],[25] Although hepatotoxicity occurs more frequently with nevirapine than efavirenz (1·4–17% versus 1.1–8% patients), drug hypersensitivity syndrome from both can cause acute/severe liver necrosis that may end fatally.[7],[26],[27],[28] However, abacavir-associated hepatic or renal failure is less common.[29] We also made similar observations in 57 (14.3%) individuals on nevirapine or efavirenz based highly active antiretroviral therapy having grade-1 hepatic toxicity as elevated serum alanine amiotranerase levels not requiring treatment discontinuation. Among them, almost 63% were aged between 31 and 45 years, 45.6% were on highly active antiretroviral therapy for ≥5 years, and men were affected one and half times more than women. In contrast, 99 (22.3%) individuals with grade-1 to grade-3 liver dysfunction initially showed improvement after highly active antiretroviral therapy suggesting that HIV infection per se may be an important cause of liver function abnormalities at least in some individuals. Similar observations have been made previously, especially during early stage of uncontrolled HIV replication or later from systemic opportunistic/concurrent infections with hepatitis B or C virus, Cytomegalovirus and Epstein Barr virus, or their therapies, autoimmune hepatitis, or alcohol abuse.[30],[31],[32] However, details of these foregoing risk factors for abnormal liver functions were not available in our study. On the other hand, preexisting anemia appears to be a significant risk factor for antiretroviral therapy-induced hepatotoxicity in this study, in contrast to a report by Ugiagbe and Eze.[30]
Long-term HIV infection itself is associated with a wide spectrum of kidney damage (focal segmental glomerulosclerosisis, cryoglobulinemia, IgA nephropathy, amyloidosis, lupus-like immune complex glomerulopathy) in 6% to 48% HIV-infected persons.[33],[34] Although no comparative Indian data could be found, young males of black races or individuals aged >40 years from other ethnic backgrounds, and preexisting diabetes or hypertension are important risk factors for HIV-associated nephropathy/chronic kidney disease before and after antiretroviral therapy.[10],[20],[21],[35],[36] Nephropathy typically presents with nephritic-range proteinuria (>3.5 g/d), azotemia, hypoalbuminemia and hyperlipidemia. Tenofovir and indinavir are well known for renal toxicity but isolated cases of nephrotoxicity with almost all HAART agents exist.[22] Highly active antiretroviral therapy for long periods is also strongly associated with increased risk for chronic kidney disease.[35],[37],[38] Antiretroviral therapy-induced renal toxicity with or without hepatotoxicity occurs in nearly 1.2% to 48% HIV-affected individuals leading to increased risk for acute renal failure, chronic kidney disease and mortality related to hemodynamic stress, volume depletion, radiocontrast or nephrotoxic drugs administration.[35],[37],[39],[40],[41] No clinical or laboratory evidence of acute kidney injury or chronic kidney disease, or intake of nephrotoxic drug (s) (itraconzole, foscarnet, amphotericin, acyclovir, aminoglycosides, etc) for HIV-related opportunistic infections, the potential risk factors for renal dysfunction, was observed in our study. However, we did not perform renal biopsy for microscopic tissue damage. On the other hand, elevated urea in 10 (2.5%) and creatinine levels in 9 (2.3%) individuals on tenofovir + lamivudine + efavirenz, tenofovir + lamivudine + nevirapine or abacavir + nevirapine regimen decreased following therapy reflecting their possible low nephrotoxic potential despite prolonged use, particularly in the absence of other risk factors.
Although 29 individuals had treatment for pulmonary tuberculosis prior to initiating highly active antiretroviral therapy and other 6 individuals developed nevirapine hypersensitivity, details of any abnormal hepatorenal functions were not available.
Limitations
The Cockcroft–Gault equation used for defining/staging chronic kidney disease is not specifically validated in the HIV affected. Viral load studies or evaluation of other risk factors precipitating hepatorenal disease/toxicity/dysfunction in HIV affected were not part of the study. An analysis of recorded biochemical parameters on yearly basis was not possible from the antiretroviral therapy records. Since antiretroviral therapy in all HIV affected was regimen based, individual drugs responsible for hepatorenal abnormalities remained unidentified. Information on concomitant infections and their treatment/prophylaxis particularly for Pneumocystis jiroveci pneumonia, viral hepatitis, as well as history of alcohol abuse in study subjects was unrecorded. Thus, hepatic or renal function abnormalities from these potential risk factors in some of the HIV-affected study subjects cannot be ruled out. A small number of subjects and retrospective data analysis were other important limitations.
Conclusions
Nevirapine or efavirenz-based regimens of variable duration caused grade-1 hepatic toxicity in some individuals while HAART improved preexisting HIV-associated hepatic or renal dysfunction in the majority of cases. Preexisting anemia remains a significant risk factor for HAART-induced hepatotoxicity in this study. However, HAART nephrotoxicity was not a significant problem. The study reflects low potential of HAART-associated hepatic or nephrotoxicity despite prolonged use, especially in the absence of other risk factors. Nevertheless, better designed prospective studies are recommended for drug wise analysis and to delineate highly active antiretroviral therapy-associated hepatorenal dysfunction or other risk factors contributing toward it.
Statement of ethics
The study was approved by Institutional Scientific Review and Ethics Committee (Regn no.- ECR/490/Inst/HP/2013/RR-16). All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2013. All patients were provided treatment and care as per standard guidelines.
Acknowledgements
The authors gratefully acknowledge the erudite help and support extended by Dr. B. S. Rana, Associate Professor of Gastroenterology and Hepatology and Dr. Pankaj Kumar, Assistant Professor of Internal Medicine, all from Dr. R. P. Govt. Medical College, Kangra (Tanda), H.P., India, in interpretation of liver and renal functions tests. We also thank Mr. Sushant Sharma of Community Medicine (Biostatistics) and the staff members at antiretroviral therapy center, Dr. R. P. Govt Medical College, Kangra (Tanda), H.P. for providing useful inputs for the study.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form, the patients or their parents/guardians have given their consent for the patient's images and other clinical information to be reported in the journal. The patient/parents/guardians understand that name and initials will not be published and due efforts will be made to conceal identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
| 1. |
National AIDS Control Organization. India HIV Estimations 2015. Technical Report. Department of AIDS Control. Ministry of Health and Family Welfare, New Delhi, India. Available from: http://www.naco.gov.in. [Last assessed on 2017 Jun 25].
[Google Scholar]
|
| 2. |
National AIDS Control Organization. State Epidemiological Fact Sheets, Vol III, Northern, Central and Eastern Region, 2017. Department of AIDS Control. Ministry of Health and Family Welfare, New Delhi, India. Available from: http://www.naco.gov.in. [Last assessed on 2017 Jun 25].
[Google Scholar]
|
| 3. |
UNAIDS. Global AIDS Response Progress Reporting Estimates. Geneva, Switzerland: UNAIDS; 2016.
[Google Scholar]
|
| 4. |
Peters PJ, Moore DM, Mermin J, Brooks JT, Downing R, Were W, et al. Antiretroviral therapy improves renal function among HIV-infected Ugandans. Kidney Int 2008;74:925-9.
[Google Scholar]
|
| 5. |
Kalyesubula R, Kagimu M, Opio KC, Kiguba R, Semitala CF, Schlech WF, et al. Hepatotoxicity from first line antiretroviral therapy: An experience from a resource limited setting. Afr Health Sci 2011;11:16-23.
[Google Scholar]
|
| 6. |
Wit FW, Weverling GJ, Weel J, Jurriaans S, Lange JM. Incidence of and risk factors for severe hepatotoxicity associated with antiretroviral combination therapy. J Infect Dis 2002;186:23-31.
[Google Scholar]
|
| 7. |
Reust CE. Common adverse effects of antiretroviral therapy for HIV disease. Am Fam Physician 2011;83:1443-51.
[Google Scholar]
|
| 8. |
Reisler R, Liou S, Servoss JC, Robbins G, Theodore D, Murphy R (ACTG Liver Diseases Focus Group). Incidence of Hepatotoxicity and Mortality in 21 Adult Antiretroviral Treatment Trials (abstract 43). 1st International AIDS Society Conference on HIV Pathogenesis and Treatment; Buenos Aires, Argentina; 2001.
[Google Scholar]
|
| 9. |
Cihlar T, Ho ES, Lin DC, Mulato AS. Human renal organic anion transporter 1 (hOAT1) and its role in the nephrotoxicity of antiviral nucleotide analogs. Nucleosides Nucleotides Nucleic Acids 2001;20:641-8.
[Google Scholar]
|
| 10. |
Servoss JC, Kitch DW, Andersen JW, Reisler RB, Chung RT, Robbins GK. Predictors of antiretroviral-related hepatotoxicity in the adult AIDS clinical trial group (1989-1999). J Acquir Immune Defic Syndr 2006;43:320-3.
[Google Scholar]
|
| 11. |
Division of AIDS (DAIDS), National Institute of Allergy and Infectious Diseases National Institutes of Health US Department of Health and Human Services. Table for Grading the Severity of Adult and Pediatric Adverse Events; 2014. Available from: http://rcc.tech-res.com/DAIDS%20RCC%20Forms/. [Last assessed on 2016 Dec 26].
[Google Scholar]
|
| 12. |
Mehta RL, Kellum JA, Shah SV, Molitoris BA, Ronco C, Warnock DG, et al. Acute kidney injury network: Report of an initiative to improve outcomes in acute kidney injury. Crit Care 2007;11:R31.
[Google Scholar]
|
| 13. |
Levey AS, Eckardt KU, Tsukamoto Y, Levin A, Coresh J, Rossert J, et al. Definition and classification of chronic kidney disease: A position statement from kidney disease: Improving global outcomes. Kidney Int 2005;67:2089-100.
[Google Scholar]
|
| 14. |
Thomas R, Kanso A, Sedor JR. Chronic kidney disease and its complications. Prim Care 2008;35:329-44, vii.
[Google Scholar]
|
| 15. |
Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron 1976;16:31-41.
[Google Scholar]
|
| 16. |
Hogan M. KDIGO Conference proposes changes to CKD classification, but not to the definition. Nephrology 2009;2:9-10.
[Google Scholar]
|
| 17. |
National Kidney Foundation. K/DOQI clinical practice guidelines for chronic kidney disease: Evaluation, classification, and stratification. Ann Intern Med 2002;39 Suppl 1:S1-266.
[Google Scholar]
|
| 18. |
Mahajan VK, Raina S, Kohli S, Gupta S, Sharma S. Cognitive impairment among persons of rural background living with human immunodeficiency virus infection on antiretroviral therapy: A study from a tertiary care centre of North India. J Neurosci Rural Pract 2016;7:S131-4.
[Google Scholar]
|
| 19. |
Chaponda M, Pirmohamed M. Hypersensitivity reactions to HIV therapy. Br J Clin Pharmacol 2011;71:659-71.
[Google Scholar]
|
| 20. |
Winston J, Deray G, Hawkins T, Szczech L, Wyatt C, Young B, et al. Kidney disease in patients with HIV infection and AIDS. Clin Infect Dis 2008;47:1449-57.
[Google Scholar]
|
| 21. |
Mallipattu SK, Wyatt CM, He JC. The new epidemiology of HIV-related kidney disease. J AIDS Clin Res 2012;Suppl 4:001.
[Google Scholar]
|
| 22. |
Berns JS, Kasbekar N. Highly active antiretroviral therapy and the kidney: An update on antiretroviral medications for nephrologists. Clin J Am Soc Nephrol 2006;1:117-29.
[Google Scholar]
|
| 23. |
Ofotokun I, Smithson SE, Lu C, Easley KA, Lennox JL. Liver enzymes elevation and immune reconstitution among treatment-naïve HIV-infected patients instituting antiretroviral therapy. Am J Med Sci 2007;334:334-41.
[Google Scholar]
|
| 24. |
Macías J, Orihuela F, Rivero A, Viciana P, Márquez M, Portilla J, et al. Hepatic safety of tipranavir plus ritonavir (TPV/r)-based antiretroviral combinations: Effect of hepatitis virus co-infection and pre-existing fibrosis. J Antimicrob Chemother 2009;63:178-83.
[Google Scholar]
|
| 25. |
Salazar JC, Cahn P, Yogev R, Negra MD, Castelli-Gattinara G, Fortuny C, et al. Efficacy, safety and tolerability of tipranavir coadministered with ritonavir in HIV-1-infected children and adolescents. AIDS 2008;22:1789-98.
[Google Scholar]
|
| 26. |
Pulido F, Torralba M. NNRTI hepatotoxicity: Efavirenz versus nevirapine. J HIV Ther 2002;7 Suppl 2:S3-16.
[Google Scholar]
|
| 27. |
Brück S, Witte S, Brust J, Schuster D, Mosthaf F, Procaccianti M, et al. Hepatotoxicity in patients prescribed efavirenz or nevirapine. Eur J Med Res 2008;13:343-8.
[Google Scholar]
|
| 28. |
Sanne I, Mommeja-Marin H, Hinkle J, Bartlett JA, Lederman MM, Maartens G, et al. Severe hepatotoxicity associated with nevirapine use in HIV-infected subjects. J Infect Dis 2005;191:825-9.
[Google Scholar]
|
| 29. |
Hetherington S, McGuirk S, Powell G, Cutrell A, Naderer O, Spreen B, et al. Hypersensitivity reactions during therapy with the nucleoside reverse transcriptase inhibitor abacavir. Clin Ther 2001;23:1603-14.
[Google Scholar]
|
| 30. |
Ugiagbe RA, Eze EU. Effect of anemia on hepatotoxicity of HAART in HIV patients in Benin city. Niger Med J 2011;52:167-72.
[Google Scholar]
|
| 31. |
Sterling RK, Chiu S, Snider K, Nixon D. The prevalence and risk factors for abnormal liver enzymes in HIV-positive patients without hepatitis B or C coinfections. Dig Dis Sci 2008;53:1375-82.
[Google Scholar]
|
| 32. |
Puoti M, Nasta P, Gatti F, Matti A, Prestini K, Biasi L, et al. HIV-related liver disease: ARV drugs, coinfection, and other risk factors. J Int Assoc Physicians AIDS Care (Chic) 2009;8:30-42.
[Google Scholar]
|
| 33. |
Fabian J, Naicker S. HIV and kidney disease in Sub-Saharan Africa. Nat Rev Nephrol 2009;5:591-8.
[Google Scholar]
|
| 34. |
Foy MC, Estrella MM, Lucas GM, Tahir F, Fine DM, Moore RD, et al. Comparison of risk factors and outcomes in HIV immune complex kidney disease and HIV-associated nephropathy. Clin J Am Soc Nephrol 2013;8:1524-32.
[Google Scholar]
|
| 35. |
Mpondo BC, Kalluvya SE, Peck RN, Kabangila R, Kidenya BR, Ephraim L, et al. Impact of antiretroviral therapy on renal function among HIV-infected Tanzanian adults: A retrospective cohort study. PLoS One 2014;9:e89573.
[Google Scholar]
|
| 36. |
Crum-Cianflone N, Ganesan A, Teneza-Mora N, Riddle M, Medina S, Barahona I, et al. Prevalence and factors associated with renal dysfunction among HIV-infected patients. AIDS Patient Care STDS 2010;24:353-60.
[Google Scholar]
|
| 37. |
Wondifraw Baynes H, Tegene B, Gebremichael M, Birhane G, Kedir W, Biadgo B. Assessment of the effect of antiretroviral therapy on renal and liver functions among HIV-infected patients: A retrospective study. HIV AIDS (Auckl) 2017;9:1-7.
[Google Scholar]
|
| 38. |
Fokunang CN, Banin AN, Kouanfack C, Ngogang JY. Evaluation of hepatotoxicity and nephrotoxicity in HIV patients on highly active anti-retroviral therapy. J AIDS HIV Res 2010;2:48-57.
[Google Scholar]
|
| 39. |
Wyatt CM, Arons RR, Klotman PE, Klotman ME. Acute renal failure in hospitalized patients with HIV: Risk factors and impact on in-hospital mortality. AIDS 2006;20:561-5.
[Google Scholar]
|
| 40. |
Ayokunle DS, Olusegun OT, Ademola A, Adindu C, Olaitan RM, Oladimeji AA. Prevalence of chronic kidney disease in newly diagnosed patients with human immunodeficiency virus in Ilorin, Nigeria. J Bras Nefrol 2015;37:177-84.
[Google Scholar]
|
| 41. |
Cailhol J, Nkurunziza B, Izzedine H, Nindagiye E, Munyana L, Baramperanye E, et al. Prevalence of chronic kidney disease among people living with HIV/AIDS in Burundi: A cross-sectional study. BMC Nephrol 2011;12:40.
[Google Scholar]
|
Fulltext Views
5,192
PDF downloads
1,769





