Translate this page into:
Azathioprine weekly pulse versus methotrexate for the treatment of chronic plaque psoriasis: A randomized controlled trial
Corresponding author: Prof. Kaushal K. Verma, Department of Dermatology and Venereology, All India Institute of Medical Sciences, New Delhi, India. prokverma@hotmail.com
-
Received: ,
Accepted: ,
How to cite this article: Verma KK, Kumar P, Bhari N, Gupta S, Kalaivani M. Azathioprine weekly pulse versus methotrexate for the treatment of chronic plaque psoriasis: A randomized controlled trial. Indian J Dermatol Venereol Leprol 2021;87:509-14.
Abstract
Background:
Methotrexate is the most commonly used drug in the treatment of psoriasis with good efficacy and safety. Recently, weekly azathioprine pulse has been shown to be effective in this disease.
Aim:
The aim of this study is to compare the effectiveness and safety of weekly pulse doses of azathioprine and methotrexate for the treatment of chronic plaque psoriasis.
Methods:
In this randomized controlled trial, 80 patients with chronic plaque psoriasis were recruited. After detailed clinical and laboratory evaluation, patients were randomized to 2 groups to receive either weekly 300 mg azathioprine (n = 40) or 15 mg methotrexate every week (n = 40) for 20 weeks, following which the response to treatment and adverse effects were assessed. The patients were then followed up every 4 weeks for 3 months to determine any relapse.
Results:
Overall, 48 (60%) patients achieved PASI 75, while 36 (45%) and 59 (73.8%) patients achieved PASI 100 and 50, respectively. On intention to treat analysis, PASI ≥ 75 was achieved in 47.5% (19/40) patients in group 1 compared to 85% (34/40) patients in group 2 (p < 0.001). However, on per protocol analysis, PASI ≥ 75 was achieved in 86% (19/22) patients in group 1 and 92% (34/37) patients in group 2 (p = 0.497). Minor clinical and biochemical adverse effects were noted in both the groups, which were comparable. One (7.7%) patient in group 1 and 4 (17.4%) in group 2 relapsed during follow-up.
Limitations:
Limitations of study include small sample size and short follow-up.
Conclusion:
Weekly azathioprine pulse appears to be beneficial in the management of chronic plaque psoriasis. However, it is less effective than weekly methotrexate. It can thus be of use as a therapeutic option in patients with contraindication to methotrexate or other similar agents in this disease.
Keywords
Azathioprine weekly pulse
methotrexate
psoriasis
Introduction
Psoriasis is a complex chronic immune disease with genetic predisposition resulting in severe impairment in quality of life. The disease has a remitting and relapsing course despite the treatment with significant morbidity. The goal of management in psoriasis is to control the disease activity with minimum treatment related side effects. Methotrexate is the most commonly used drug in the treatment of moderate to severe psoriasis which has shown good efficacy and side effect profile. Recently, azathioprine in its weekly doses has been shown to be effective in the management of psoriasis.1
In this study, we evaluated and compared the therapeutic effectiveness of weekly pulse doses of azathioprine and methotrexate for the treatment of chronic plaque psoriasis and monitored the side effects of both these regimens, clinically and biochemically.
Methods
Sample size calculation
A sample size of 38 per group was required to achieve 30% difference in the PASI 75 at 20 weeks between azathioprine and methotrexate for the management of chronic plaque psoriasis with 5% alpha and 80% power.1,2
Patient recruitment
A total of 112 consecutive patients with chronic plaque psoriasis were screened, of which 80 patients meeting the inclusion criteria, which included presence of the disease for more than 6 months, psoriasis area severity index (PASI) >10 and body surface area (BSA) more >10% were enrolled. Pregnant/lactating women, patients with major systemic illness of respiratory, cardiac, renal, hepatic and gastrointestinal system, intake of immunosuppressive drugs within last 4-weeks, PASI <10, BSA <10%, hypersensitivity to azathioprine or methotrexate were excluded.
The study was approved by the Institutional Ethics Board and was funded by Indian Council of Medical Research. All participants provided the written informed consent, after a detailed discussion about the treatment plan, expected outcome, benefits and risk of treatment.
Pretreatment evaluation
A detailed history and clinical evaluation were undertaken during the first visit. Psoriasis area severity index (PASI) score and the body surface area (BSA) involved in each patient were determined to assess the severity of the disease. Pre-treatment clinical photographs of the affected sites were taken.
In each patient, baseline investigations consisting of complete blood count, erythrocyte sedimentation rate, blood glucose (fasting), serum electrolytes, renal and liver function tests, routine and microscopic examination of urine and stool, chest X-ray and electrocardiogram were done. The patients already receiving any systemic therapy were given a wash off period of 4 weeks before starting them on treatment. Prior estimation of TPMT (thiopurine methyltransferase) levels was not done due to lack of facilities.
Randomization of treatment arms
All eligible patients with normal baseline investigations were randomly allocated to two groups, group 1 and 2 [Figure 1]. Brown opaque envelopes containing 6 tablets of either azathioprine (50 mg each) or methotrexate (2.5 mg each) were prepared as per computer generated random number table. The randomization was done by another consultant in the department who was not associated with the study. A test dose of one tablet of each drug was given to the patients of respective groups and they were observed clinically for a period of 48 hours for any side effects. Following which, group 1 patients were given azathioprine 6 tablets (300 mg) and the group 2 patients were given methotrexate 6 tablets (15 mg) after meal once every week for a period of 20 weeks or till PASI 100 improvement and BSA zero were achieved, whichever earlier. Topical petrolatum jelly and oral cetirizine hydrochloride 10 mg daily for symptomatic relief were given to patients in both the groups.

- Study flow chart showing randomization, treatment assignment, follow-up and analysis
Follow-up
The patients were followed up at week 2 and 4, and then every 4 weeks while on treatment. The evaluation was done by a single blinded observer. Clinical and biochemical assessments (hemogram, liver and renal function tests) were repeated every 4 weeks to monitor the clinical response and side-effects. At the end of 20 weeks or earlier, if there was complete remission (PASI 100 improvement and BSA 0), the treatment was stopped. Post-treatment photographs were taken for evaluation of visual outcome and comparison in each patient. All baseline investigations including chest X-ray and electrocardiogram were repeated at the end of treatment.
The patients were then followed up every 4 weeks for a period of 3 months following the completion of treatment to determine any relapse of the disease. A patient was considered to have relapsed if there was loss of 50% or more of PASI improvement from the baseline who has achieved a significant clinical response.
Statistical analysis
Data analysis was carried out using Stata 12.0 (College Station, Texas, USA. Data were presented as number (%) or mean ± SD/ median (min-max) as appropriate. Baseline categorical and continuous variables were compared using Chi-square test and independent t test/wilcoxon ranksum test respectively. Both intention-to-treat and per protocol analysis was carried out for the primary outcome and the response to treatment were compared using Chi-square test and results were reported as difference in response rate (95% CI) along with odds ratio (95%CI) after adjustment with age, gender, duration, weight and baseline PASI. For patients lost to follow-up, an outcome of PASI <75 was assumed to calculate odds ratio in intention to treat analysis. The secondary and adverse events were compared between the groups using Fisher’s exact test. The P value less than 0.05 was considered statistically significant.
Results
A total of 80 patients (64 male and 16 female), between 16-62 years of age (mean: 36.37 ± 13.5 years), were included in the study from September 2010 to August 2014. The demographic profile of the patients is shown in Table 1. Mean PASI in group 1 was 15.09 (10.1-27.3, S.D.=4.78) while it was 17.83 (10.2-39.1, S.D.=7.20) in group 2 (p = 0.11). Most of the patients have received methotrexate in the past for variable duration with remitting relapsing course of disease. They were randomized into 2 groups with 40 patients each in group 1 and group 2 [Figure 1]. Of these 80 patients, 59 (73.8%) patients completed the treatment (22 patients in group 1 and 37 in group 2). Twenty-one (26.3%) patients were lost to follow-up during the treatment period, out of which 18 patients were in group 1 and 3 patients were in group 2. Seven patients in group 1 stopped the treatment because of the slow response to the therapy, 5 due to gastric intolerance, 3 due to inability to follow-up, while 3 patients could not be contacted despite repeated attempts. In group 2, one patient discontinued the therapy because of gastric intolerance, while 2 patients could not follow-up because of long distance. The treatment had to be discontinued in 5 (6.3%) patients of which 2 patients had severe nausea, vomiting and abdominal discomfort (group 1), 2 patients because of worsening disease activity and one patient had pregnancy (group 1).
| Profile | Group 1 (n=40) | Group 2 (n=40) | P |
|---|---|---|---|
| Gender, n (%) | |||
| Male | 32 (80) | 32 (80) | 1.000 |
| Female | 8 (20) | 8 (20) | |
| Age* (years) | 36.3±13.5 | 36.4±12.6 | 0.9728 |
| Disease#, duration (years) | 7 (0.16-30) | 6 (0.2-25) | 0.3095 |
| Weight* | 69.2±14.8 | 65.1±12.8 | 0.1931 |
| Marital status, n (%) | |||
| Married | 29 (72.5) | 27 (67.5) | 0.626 |
| Unmarried | 11 (27.5) | 13 (32.5) |
Response to treatment
The response to treatment was excellent (PASI 100) in 36 (45%) patients of which 14 (38.9%) patients were in group 1 and 22 (61.1%) in group 2, very good (PASI 75) in 48 (60%) patients {17 (35.4%) patients in group 1 and 31 (64.9%) in group 2} and good (PASI 50) in 59 (73.8%) patients {22 (37.3%) patients in group 1 and 37 (62.7%) in group 2} [Table 2 and Figures 2, 3]. Mean PASI at 20 weeks was 1.9 (0-9.9) in group 1 while it was 0.89 (0-5.8) in group 2.
| Intention to treat analysis | ||||
|---|---|---|---|---|
| Response | Group 1 (n=40), n (%) | Group 2 (n=40), n (%) | Difference (95% CI) | OR (95% CI) |
| PASI ≥75 | 19 (47.5) | 34 (85) | 37.5 (5.0-70.0) | 6.3 (2.2-18.2) |
| PASI <75 | 21 (52.5) | 6 (15) | 6.6 (2.1-20.7)* | |
| Per protocol analysis | ||||
| Response | Group 1 (n=22), n (%) | Group 2 (n=37), n (%) | Difference (95% CI) | OR (95% CI) |
| PASI ≥75 | 19 (86.4) | 34 (91.9) | 5.5 (−11.3-22.3) | 1.8 (0.33-9.8) |
| PASI <75 | 3 (13.6) | 3 (8.1) | 1.4 (0.19-10.2)* | |

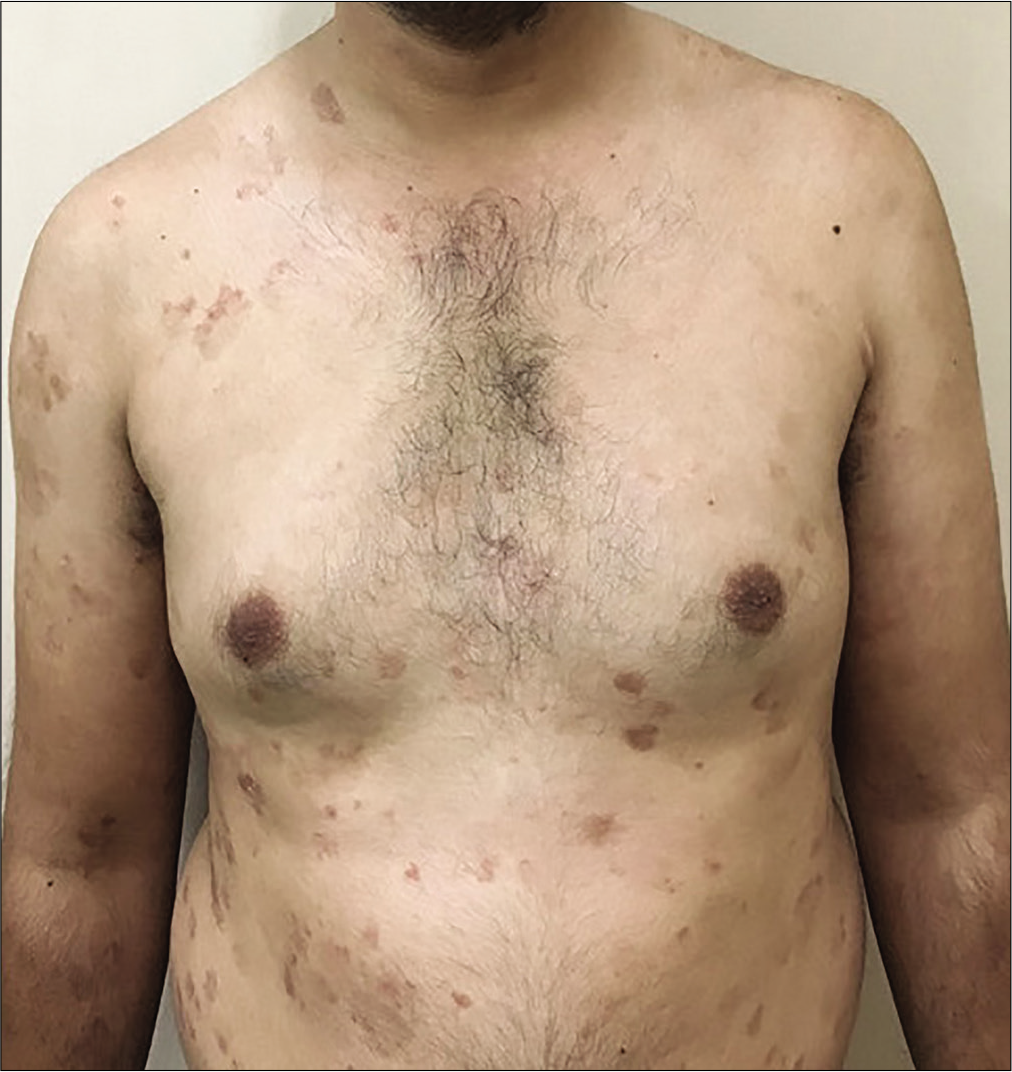
- Patient receiving azathioprine 300 mg weekly pulses with psoriasis area severity index 16.4 at baseline
- Same patient showing reduction in psoriasis area severity index to 4.8 at 3rd follow-up visit
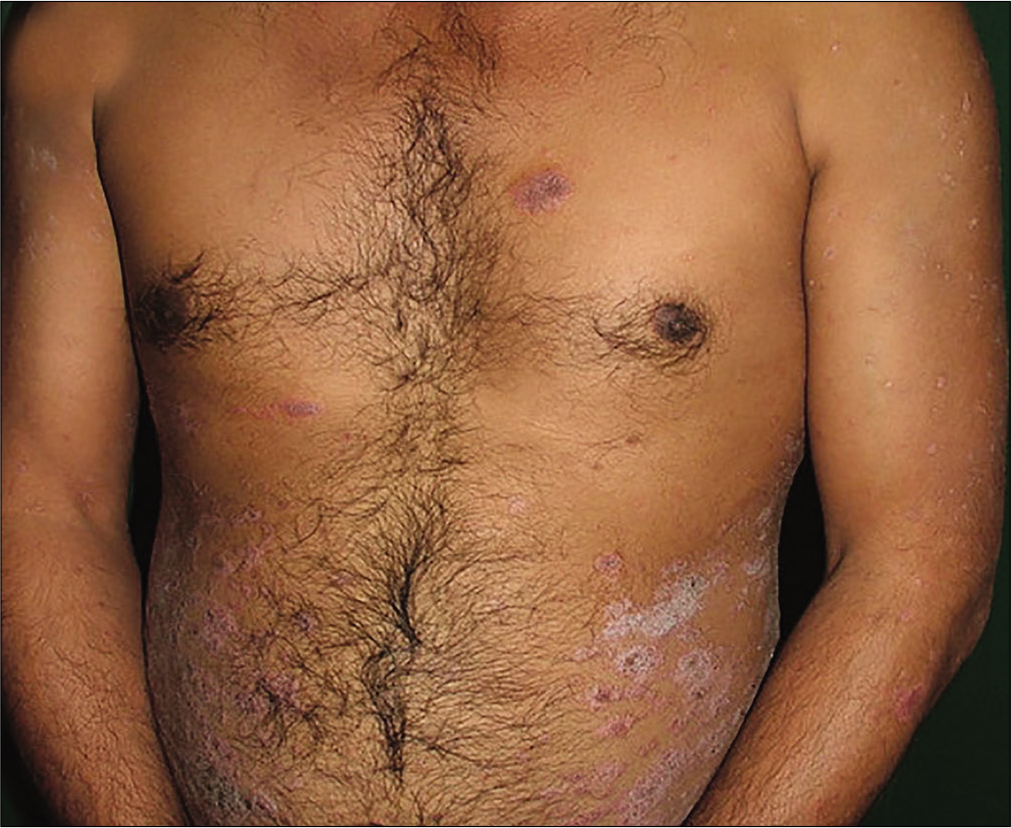
- Patient receiving weekly 15 mg methotrexate with psoriasis area severity index 17.4 at baseline
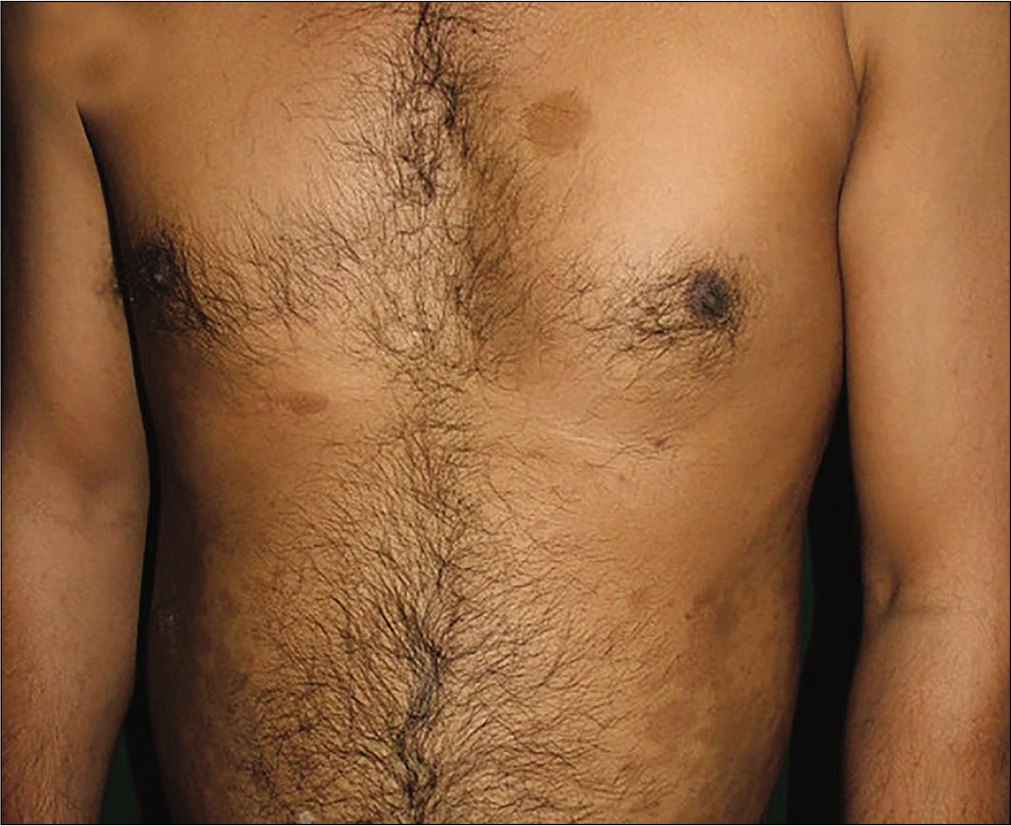
- Same patient showing reduction in psoriasis area severity index to 0 at 3rd follow-up visit
Intention to treat analysis
On intention to treat analysis, PASI ≥ 75 was achieved in 47.5% in group 1 compared to 85% patients in group 2, and the difference was statistically significant (p < 0.001) showing superiority of treatment outcome in the methotrexate group.
Per protocol analysis
On per protocol analysis, PASI ≥ 75 was achieved in 86% (19/22) patients in group 1 and 92% (34/37) patients in group 2 and which was comparable (p = 0.497) indicating equal efficacy of 2 treatment regimens.
Adverse effects
The clinical adverse effects were noted in both the groups during the treatment which were managed conservatively. The adverse effects were comparable in two groups and were statistically not significant [Table 3]. The biochemical parameters remained within normal range in the majority of the patients. In some patients, there were transient changes in hemoglobin, platelet count, total leukocyte count, serum bilirubin and liver enzymes, which returned to normal during the course of treatment.
| Adverse effect | Group 1 (n=40), n (%) | Group 2 (n=40), n (%) | P |
|---|---|---|---|
| Nausea | 18 (45) | 14 (35) | 0.361 |
| Vomiting | 12 (30) | 6 (15) | 0.108 |
| Diarrhea | 4 (10) | 1 (2.5) | 0.359 |
| Secondary infection | |||
| Bacterial | 5 (12.5) | 1 (2.5) | 0.201 |
| Fungal | 1 (2.5) | 0 | 1 |
| Abdominal pain | 1 (2.5) | 7 (17.5) | 0.057 |
| Psychological manifestation | 0 | 1 (2.5) | 1.000 |
| Others | 10 (25.0) | 15 (37.5) | 0.228 |
Post-treatment follow-up and relapse
Thirteen patients in group 1 and 23 patients in group 2 completed the post treatment follow-up of 3 months. One patient (7.7%) in group 1 and 4 patients (17.4%) in group 2 relapsed during follow-up.
Discussion
Methotrexate remains the most frequently used drug in the treatment of psoriasis despite availability of several therapeutic agents particularly in the developing world. Azathioprine, a potent T cell inhibitor, has been sparingly used in this disease. Both the drugs have shown their value in the treatment of psoriasis, though there is paucity of data comparing their efficacy and safety.
In most studies on psoriasis, azathioprine has been used in a dose of 2-4mg/kg/day once daily. Munro treated 29 patients of psoriasis with azathioprine 2–4 mg/kg/day; of which, 19 (65%) showed significant improvement but 7 (24.1%) had treatment failure.3 Greaves et al. treated 10 patients with disabling methotrexate nonresponsive psoriasis, using azathioprine 2.5 mg/kg/day for 6 months. Five (50%) of these patients had > 25% improvement at the end of 6 months.4
Verma et al. have shown efficacy of weekly 300 mg azathioprine pulse therapy in the management of chronic plaque psoriasis. 1 In an open-label clinical trial, 50 patients of chronic plaque psoriasis were treated with a 300 mg bolus dose of azathioprine given once every week orally for 24 weeks. A total, 28 (56%) patients completed the treatment of which 21 (42%) achieved PASI 75. However, therapy had to be withdrawn in 5 (10%) patients due to adverse effects.
Methotrexate is traditionally used once a week, usually 15 mg dose, in the management of plaque psoriasis. In a recent meta-analysis of 11 studies showing efficacy of methotrexate in psoriasis, PASI 75 was achieved in 45.2% patients and treatment limiting side effects were noted in 6.9 ± 1.4% cases.2 Kumar et al. reviewed 197 patients with psoriasis treated with once-weekly oral MTX (0.3–0.5 mg/kg/week) in a retrospective study. More than 75% improvement was noted in 88% patients in 8.5 ± 5.1 weeks. 5 However, some randomized controlled trials comparing efficacy of methotrexate to biologics have shown weekly methotrexate treatment resulting in a PASI 75 improvement in 35.5%-42% patients.6-8
Few studies have compared the efficacy of weekly methotrexate and azathioprine, though, there are no studies comparing weekly azathioprine pulse treatment with weekly methotrexate. In a randomized controlled trial, Maliq et al. treated 50 patients of psoriasis, comparing azathioprine 1.5–3 mg/kg/day with methotrexate 10 mg/week, given for 8 weeks. There was an excellent response in 5 (27%) patients in the azathioprine group and 13 (73%) in the methotrexate group, with reduction in PASI of ≥ 80, thus showing superior efficacy of methotrexate. 9 Mezzadara treated 20 patients of psoriasis using 6 g of azathioprine over a period of 18 days, and found it comparable with methotrexate.10
In our study, the response to treatment as determined by reduction in PASI score, on intention to treat analysis, PASI >75 was achieved in 47.5% (19/40) patients in group 1 and in 85% (34/40) patients in group 2 (p = 0.001), indicating a better response in group 2 (methotrexate group). However, on per protocol analysis, PASI 75 was achieved in 86% (19/22) patients in group 1 and 92% (34/37) patients in group 2, which was comparable in two groups (p = 0.497).
The response noted in our study for both the group is comparable to previous study done by us and Kumar et al.1,5 The response to azathioprine was superior to that noted by Maliq et al., though the response to methotrexate was again comparable.9
There was a high drop out in group 1 which was similar to our previous study.1 The high dropout in azathioprine group is attributed to its slow rate of response compared to methotrexate. This limits its utility in patients with extensive disease requiring rapid onset of action. Patients in both the groups had mild adverse effects which were comparable in two groups and were statistically not significant. Similar adverse effects were reported in the previous studies also with two drugs.1-10 In our study, TPMT (thiopurine methyltransferase) determination was not performed because of lack of facilities. Also, it has been shown that prior estimation of TPMT does not predict azathioprine induced side effects and a routine clinical, hematological and biochemical evaluation is adequate for follow-up monitoring.11
Thirteen patients in group 1 and 23 patients in group 2 completed post-treatment follow-up of three months of which 1 (7.7%) patient in group 1 and 4 (14.4%) patients in group 2 relapsed. Singh et al. reported relapse in 36.5% in patients after 12 weeks of stopping methotrexate which is higher compared to patients in methotrexate group in our study and much higher than our azathioprine group patients.12 We noted relapse in 2 patients (4%) during post-treatment follow-up at 8 and 12 weeks, respectively, in our previous study with pulse azathioprine which was less compared to the current study.1 Greaves et al. noted relapse in two (20%) patients within one month of stoppage of azathioprine treatment, which is also higher compared to our pulse azathioprine group suggesting possibility of longer lasting remission in patients treated with weekly pulse doses of azathioprine.3
Limitations
Limitations of this study include small sample size and short follow-up.
Conclusion
Our study has thus shown that weekly azathioprine pulse appears to be beneficial in the management of chronic plaque psoriasis. It is however less effective than weekly methotrexate. Also, the dropout rates were higher in this group, possibly due to longer therapeutic response time and gastrointestinal side effects. Weekly azathioprine pulse thus can be of use as a therapeutic option in patients with contraindication to methotrexate or other similar agents in this disease. Further studies, however, are needed to confirm our findings.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Indian Council of Medical Research funded this project.
Conflicts of interest
There are no conflicts of interest.
References
- Effectiveness of weekly azathioprine pulse in the treatment of chronic plaque psoriasis: An open-label study. Clin Exp Dermatol. 2016;41:717-22.
- [CrossRef] [PubMed] [Google Scholar]
- Safety and efficacy of methotrexate in psoriasis: A meta-analysis of published trials. PLoS One. 2016;11:E0153740.
- [CrossRef] [PubMed] [Google Scholar]
- Short-term methotrexate therapy in psoriasis: A study of 197 patients. Int J Dermatol. 2002;41:444-8.
- [CrossRef] [PubMed] [Google Scholar]
- Efficacy and safety results from the randomized controlled comparative study of adalimumab vs. methotrexate vs. placebo in patients with psoriasis (CHAMPION) Br J Dermatol. 2008;158:558-66.
- [CrossRef] [PubMed] [Google Scholar]
- Efficacy and safety of infliximab vs. methotrexate in patients with moderate-to-severe plaque psoriasis: Results of an open-label. active-controlled, randomized trial (RESTORE1) Br J Dermatol. 2011;165:1109-17.
- [CrossRef] [PubMed] [Google Scholar]
- A 52-week trial comparing briakinumab with methotrexate in patients with psoriasis. N Engl J Med. 2011;365:1586-96.
- [CrossRef] [PubMed] [Google Scholar]
- Comparison of methotrexate and azathioprine in the treatment of psoriasis: A randomized controlled trial. J Pak Assoc Dermatol. 2010;20:152-7.
- [Google Scholar]
- Therapeutic experience with azathioprine in psoriasis. G Ital Dermatol Minerva Dermatol. 1972;47:72-6.
- [Google Scholar]
- Thiopurine methyltransferase enzyme activity determination before treatment of inflammatory bowel disease with azathioprine: Effect on cost and adverse events. Can J Gastroenterol. 2005;19:147-51.
- [CrossRef] [PubMed] [Google Scholar]
- Relapse in psoriasis with two different tapering regimens of methotrexate: A randomized open-label controlled study. Indian J Dermatol Venereol Leprol. 2015;81:144-7.
- [CrossRef] [PubMed] [Google Scholar]






