Translate this page into:
Chemical leukoderma due to hydroquinone: An unusual phenomenon
2 Department of Dermatology, Central Government Health Service, Kolkata, West Bengal, India
3 Department of Dermatology, Katihar Medical College and Hospital, Katihar, Bihar, India
Correspondence Address:
Anupam Das
Building - “Prerana” 19, Phoolbagan, Kolkata - 700 086, West Bengal
India
| How to cite this article: Das A, Ghosh A, Kumar P. Chemical leukoderma due to hydroquinone: An unusual phenomenon. Indian J Dermatol Venereol Leprol 2019;85:567 |
Sir,
Hydroquinone is a commonly prescribed drug for postinflammatory hyperpigmentation, melasma and various other indications. The commonly reported adverse effects include irritant contact dermatitis and exogenous ochronosis. However, in rare circumstances, it can lead to permanent leukoderma. Here, we present a case of a middle-aged female who developed depigmentation over the face following unsupervised application of 4% hydroquinone.
A 35-year-old female patient presented with white patches over her face. She was applying hydroquinone 4% cream for melasma since February 2015 on the advice of a local chemist. Eight months after continued application, she started developing the lesions (from October 2015). Initially, she developed white macules over the right cheek, which increased in number and size for three months, and thereafter, the lesions acquired the present status and became static. There were no features suggestive of koebnerization. Cutaneous examination showed depigmented macules coalescing to form patches typically restricted to the areas where lesions of melasma were present (forehead, cheeks and chin) [Figure - 1]; similar lesions were not present elsewhere in the body. There was erythema overlying the depigmented patches on the forehead and left cheek, attributed to the use of multiple over-the-counter preparations. Skin biopsy was done from a depigmented macule, and histopathlogy showed blunt rete ridges, melanophages in dermis and perivascular chronic inflammatory infiltrate [Figure - 2] and [Figure - 3]. The biopsy findings were consistent with chemical leukoderma. The patient was diagnosed to be suffering from depigmentation caused by hydroquinone. She is currently under treatment (0.1% tacrolimus and sunscreen usage).
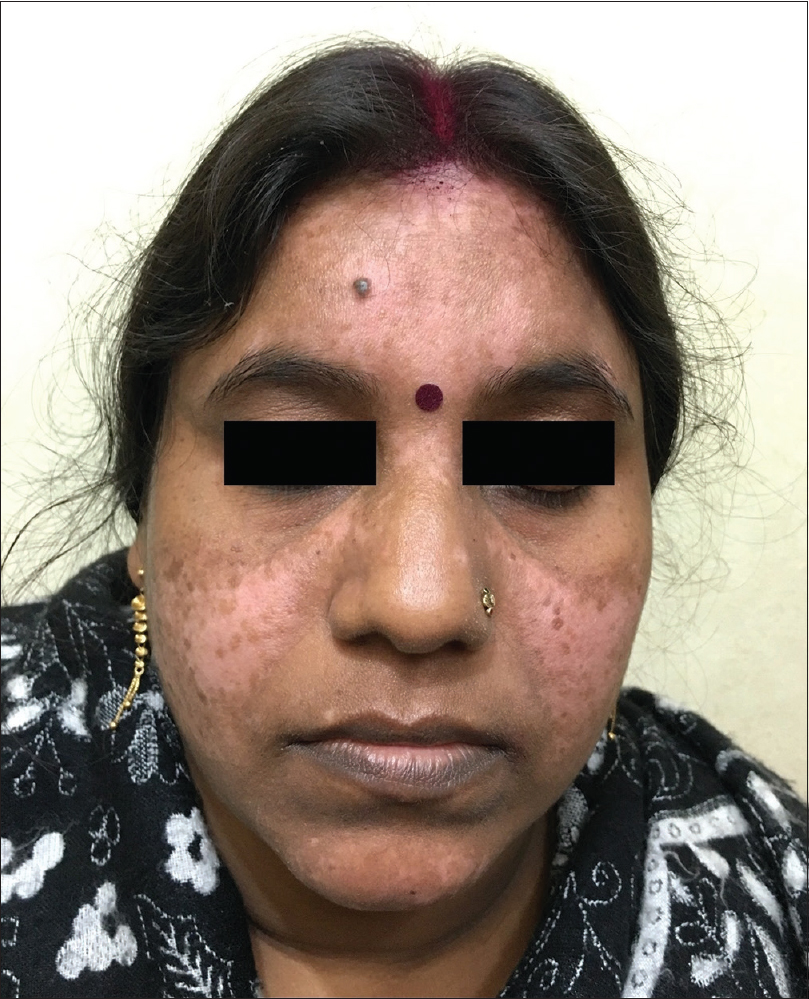 |
| Figure 1: Depigmented macules and patches over forehead, cheeks and chin |
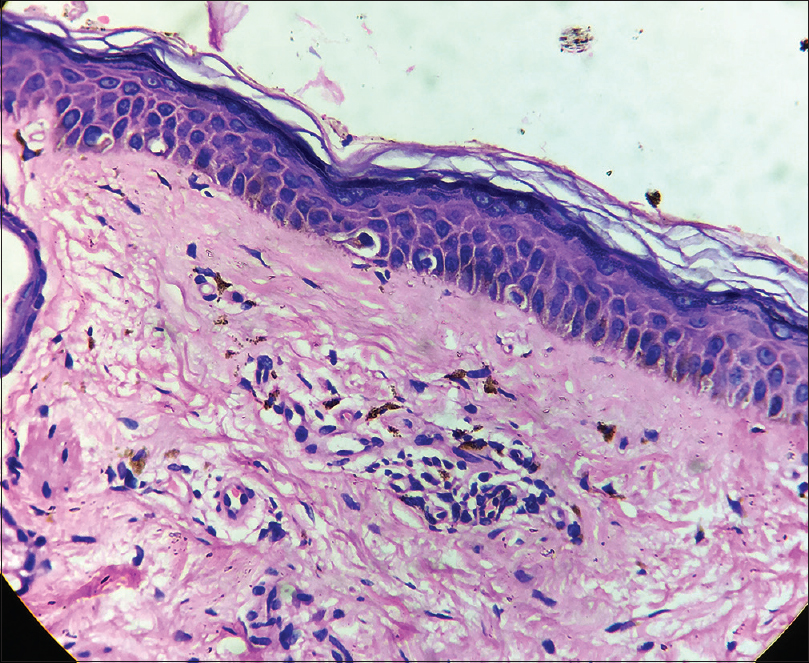 |
| Figure 2: Blunt rete ridges and melanophages in dermis (H and E, ×100) |
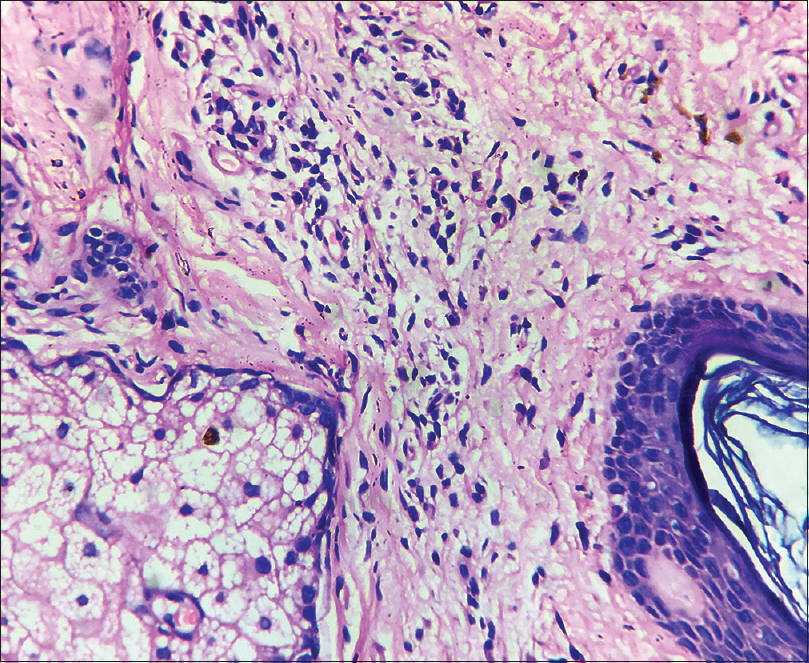 |
| Figure 3: Perivascular chronic inflammatory infiltrate (H and E, ×400) |
Oettel was the first to note the activity of hydroquinone as a bleaching agent. In 1961, Spencer reported the first clinical trial with hydroquinone as a bleaching agent. However, on 29 August 2006, US Food and Drug Administration banned all hydroquinone skin-bleaching products that were not approved through the new drug application process. This was done because of increasing concerns of possible carcinogenicity and disfiguring exogenous ochronosis. The mechanism of action of depigmentation is varied, including inhibition of tyrosinase enzyme, direct cytotoxic effect on melanocytes, degradation of melanosomes and inhibition of replication of DNA and transcription to RNA.
Adverse effects of hydroquinone include irritant contact dermatitis, hyperpigmentation, depigmentation, conjunctival melanosis, corneal degeneration, nail discoloration and exogenous ochronosis. Irritant contact dermatitis is the most common adverse effect and it is characterized by erythema, pruritus, mild edema, burning and scaling. The probability increases on using 4% hydroquinone in comparison to 2% hydroquinone, and the chances are high irrespective of the preparation (monotherapy or combination).[1]
Hyperpigmentation of skin is another complication more commonly reported with 4% hydroquinone and preferentially affects individuals with Fitzpatrick skin types V and VI. This should be identified precisely because it necessitates the withdrawal of hydroquinone. If the treatment continues, hydroquinone-induced hyperpigmentation worsens.
Conjunctival melanosis, corneal degeneration and nail discoloration are rarest side effects and are not common with topical hydroquinone preparations.
Exogenous ochronosis is a worrisome adverse effect and is characterized by gray-brown or blue-black macules with hyperchromic, pinpoint and caviar-like papules. Diagnosis of this condition requires a high index of suspicion, and the hallmark histopathological feature is the presence of the ochre-colored, banana-shaped fibers in the dermis.
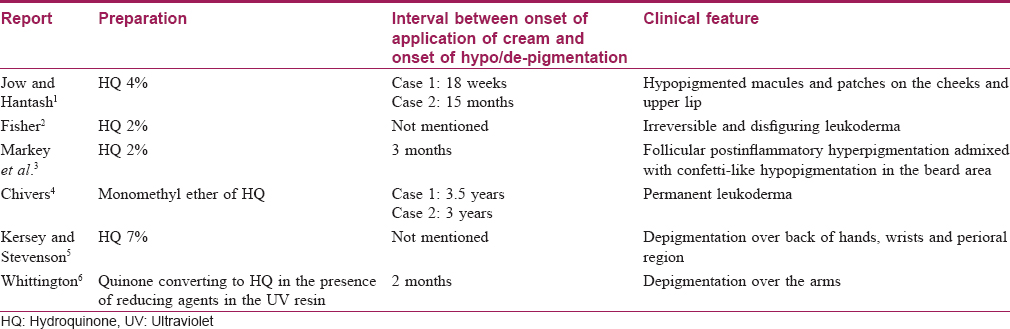
Depigmentation of skin (consistent with our case) is a rare adverse effect, clinically manifesting as confetti-like hypopigmented and depigmented macules. It was initially reported to occur with monobenzyl ether of hydroquinone. However, monomethyl ether of hydroquinone and native hydroquinone have also emerged as contributing factors. [Table - 1] summarizes the reports of depigmentation caused by hydroquinone-containing preparations. The mechanism of action behind this adverse effect could be related to the selective cytotoxic effect of hydroquinone towards melanocytes and related cells.[7]
Previous reports of hydroquinone-induced hypopigmentation and depigmentation have been confined to African and American population. To our knowledge, this is the first case of hydroquinone-induced depigmentation from the Indian subcontinent. Based on our report and previous documentations, it is quite evident that permanent leukoderma can be a rare side effect of hydroquinone-containing preparations, and this should be kept in mind while prescribing any cream or ointment formulation containing hydroquinone of any strength: the duration of use must be strictly limited as per the requirement. It is proposed that the market should limit the concentration of hydroquinone to a maximum of 4%, and these should only be sold with a prescription and be used under careful supervision by a dermatologist.[8]
Acknowledgement
The authors would like to sincerely thank Prof. M. Ramam and Prof. Phillip Mckee for their valuable help in clinico-pathological correlation in this case.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form, the patient has given her consent for her images and other clinical information to be reported in the journal. The patient understands that name and initials will not be published and due efforts will be made to conceal identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
The biopsy was done as a routine OPD procedure and no other investigations were required
Conflicts of interest
There are no conflicts of interest.
| 1. |
Jow T, Hantash BM. Hydroquinone-induced depigmentation: Case report and review of the literature. Dermatitis 2014;25:e1-5.
[Google Scholar]
|
| 2. |
Fisher AA. Leukoderma from bleaching creams containing 2% hydroquinone. Contact Dermatitis 1982;8:272-3.
[Google Scholar]
|
| 3. |
Markey AC, Black AK, Rycroft RJ. Confetti-like depigmentation from hydroquinone. Contact Dermatitis 1989;20:148-9.
[Google Scholar]
|
| 4. |
Chivers CP. Two cases of occupational leucoderma following contact with hydroquinone monomethyl ether. Br J Ind Med 1972;29:105-7.
[Google Scholar]
|
| 5. |
Kersey P, Stevenson CJ. Vitiligo and occupational exposure to hydroquinone from servicing self-photographing machines. Contact Dermatitis 1981;7:285-7.
[Google Scholar]
|
| 6. |
Whittington CV. Hypopigmentation from UV resin additive. Contact Dermatitis 1981;7:289-92.
[Google Scholar]
|
| 7. |
Levitt J. The safety of hydroquinone: A dermatologist's response to the 2006 federal register. J Am Acad Dermatol 2007;57:854-72.
[Google Scholar]
|
| 8. |
Tse TW. Hydroquinone for skin lightening: Safety profile, duration of use and when should we stop? J Dermatolog Treat 2010;21:272-5.
[Google Scholar]
|
Fulltext Views
12,834
PDF downloads
2,904





