Translate this page into:
Clinical and bacteriological profile and outcome of sepsis in dermatology ward in tertiary care center in New Delhi
2 Department of Medicine, All India Institute of Medical Sciences, New Delhi 110 029, India
3 Department of Microbiology, All India Institute of Medical Sciences, New Delhi 110 029, India
Correspondence Address:
V K Sharma
Department of Dermatology and Venereology, All India Institute of Medical Sciences, New Delhi 110 029
India
| How to cite this article: Asati D P, Sharma V K, Khandpur S, Khilnani G C, Kapil A. Clinical and bacteriological profile and outcome of sepsis in dermatology ward in tertiary care center in New Delhi. Indian J Dermatol Venereol Leprol 2011;77:141-147 |
Abstract
Background: There is paucity of data regarding the clinical and bacteriological profile of sepsis in dermatology in-patients. Aims: To study the frequency, etiology, and outcome of sepsis dermatology in-patients. Methods: The study was conducted in a 30-bedded dermatology ward of a tertiary care center. Sepsis was defined by presence of ≥2 SIRS (systemic inflammatory response syndrome) criteria along with evidence of infection (clinically obvious/culture proven infection of skin or internal organs). Patients were also assessed for known (common) risk factors of sepsis. In suspected sepsis patients, at least two samples of blood cultures by venepuncture were taken. Pus, skin swab, urine, and sputum samples were also collected for culture as needed with avoidance of contamination. Results: Among 860 admitted patients studied from November 2004 to July 2006, 103 (12%) fulfilled SIRS criteria. Of these, 63 had nonsepsis causes of SIRS positivity, while 40 (4.65%) had sepsis. Majority of the sepsis patient had vesicobullous diseases (42.5%), erythroderma (25%), toxic epidermal necrolysis (TEN) (22.5%). Severe sepsis developed in 17 (42.5%) patients, while 15 (37.5%) died. Methicillin-resistant Staphylococcus aureus (MRSA) was the commonest organism isolated (99; 25.9%) in all culture specimens followed by Acinetobacter spp. (52; 13.6%), Pseudomonas spp. (40; 10.5%), Methicillin-sensitive S. aureus (MSSA: 33; 8.7%), and Klebsiella spp. (22; 5.8%). Various risk factors affecting mortality and sensitivity patterns for various isolates were also analyzed. Conclusion: Sepsis occurred in 40 (4.65%) inpatients in dermatology ward. The frequency of sepsis was highest in TEN (90%), followed by drug-induced maculopapular rash (20.0%), erythroderma (17.5%), and vesicobullous diseases (8.5%). MRSA, acinetobacter, pseudomonas, MSSA, and Klebsiella were important etiological agents involved in sepsis in dermatology in-patients.Introduction
Sepsis is one of the most dreaded complications in dermatology in-patients especially in patients with erythroderma, toxic epidermal necrolysis (TEN), and vesicobullous diseases. [1],[2],[3] High dose steroids and immunosuppressives add to their susceptibility to develop sepsis. [3],[4] It is important to recognize the clinical and microbial profile of sepsis in order to formulate management guidelines suitable to dermatology in-patients. Several studies have been conducted in intensive care units, burn wards, medical and surgical wards but as far as ascertained; no such study has been undertaken in the dermatology patients. [5],[6],[7] Hence, a prospective study was undertaken to assess the frequency, microbial etiology and outcome of sepsis in the dermatology ward.
Methods
All patients admitted in the Dermatology ward during the study period of 20 months (November 22, 2004 to July 26, 2006) were screened for sepsis as defined by fulfillment of the following criteria: systemic inflammatory response syndrome (SIRS). [8] The methodology has been described in the preliminary report. [9] SIRS was defined as presence of two or more of the following: (a) fever {oral temperature > 38 o C} or hypothermia {<36 o C}, (b) tachypnea {>20 breaths per min} or PaCO 2 lower than 32 torr, (c)tachycardia {>90 HR per min}, (d) leucocytosis {>12 000/μl}, leucopenia <4000/μl} or ≥ 10% "band cells" {immature neutrophils}.
PLUS
Presence of infection as suggested by any one of the following:
- abscess, crusting pyoderma or other evident focus of infection,
- clinical or radiological evidence of pneumonia,
- positive blood culture,
- any other positive culture, e.g.; urine, sputum, etc.
In this prospective study, all the admitted patients were closely monitored and whenever they were found to have positive SIRS features, an active search for clinical/culture proven source of infection was performed. In the absence of documented infection, other inflammatory conditions like erythroderma, severe arthritis, sterile pustular eruptions of psoriasis or type 2 lepra reaction were attributed as the cause of SIRS positivity and these patients were excluded from the study. The differentiation between sepsis and non-sepsis patients was done along this criteria and relevance of an infection to patients′ sickness was decided after expert opinion of dermatologists, one senior physician and a microbiologist about the patient′s "septic look" clinically.
Bacteremia was defined as isolation of one or more microorganisms on blood culture in association with SIRS positivity. Two consecutive positive blood cultures with identical susceptibility profiles were essential to qualify for bacteremia for known skin contaminants. Nosocomial (or hospital-acquired) infection was considered when no infection was present at hospitalization, but developed 48 h or later. Severe sepsis was considered as presence of one or more organ dysfunction i.e. metabolic acidosis, acute encephalopathy, oliguria, hypoxemia, disseminated intravascular coagulation, or persistent hypotension. [8]
In all the patients, a detailed clinical history and detailed physical examination was followed by hematological, biochemical, and other relevant investigations. Samples for blood and other cultures (as relevant in each patient) were collected under aseptic conditions. All samples were taken preferably at admission or before the starting of antibiotics and antifungal agents. If the patient had persistent or new symptoms/signs of sepsis, the cultures were repeated again. Blood culture sample was taken into brain−heart infusion broth, then inoculated on 5% sheep blood agar, incubated overnight at 37 ºC aerobically in McConkey′s agar and in presence of 5% CO 2 (blood agar). Bacterial pathogens were identified by standard microbiological techniques. [9],[10] Antimicrobial sensitivity was performed on Muller−Hinton agar (Hi-media India) by the standard disk diffusion method recommended by the National Committee for Clinical Laboratory Standards (NCCLS). [10] Relevance of a positive culture was determined by its significance in the individual clinical setting and also by its reproducibility. Two consecutive positive blood cultures with identical susceptibility profiles were essential to qualify for bacteremia for known skin contaminants like coagulase-negative staphylococci, micrococci, corynebacteria, or diptheroids. HIV status was not routinely done as most of patients had evident skin disease and there was no history of high-risk behavior.
The sepsis patients were managed with the help of internal medicine, nephrology and hematology departments but stayed in dermatology ward isolation rooms till recovery or death. In advanced stages of sepsis with disseminated intravascular coagulopathy (DIC), hypotension, dyspnea, and multiorgan failure, patients were managed with active help of other specialities. All possible treatment needed for such a sick patient including ventilators were provided in dermatology wards.
The study was approved by the Ethics Committee of our Institute. Written informed consent was taken from the patient or his/her guardian about inclusion of the patient in the study.
Statistical analysis
Descriptive statistics, i.e.; frequency distribution and tabulation percentages were calculated for the categorical variables. Mean and standard deviations were calculated for the continuous variables. To study the association of the probable categorical/qualitative risk factors for mortality; Chi-square test or Fisher′s exact test was applied wherever applicable. The comparison between outcome with two levels and continuous variables was carried out by using t-test. The continuous variables, which were much skewed, were transformed by logarithmic transformation. A value of less than 0.05 was considered for statistical significance level.
Results
Profile of admitted patients
A total of 860 patients were admitted during the study period, which included 458 (53.26%) males and 402 (46.74%) females, with mean age of 36.56 years ± 23.76 years (range 1−90 years). One hundred and three patients (12.0%) fulfilled SIRS criteria. Of these, 63 (61.2%) were nonsepsis by lack of focus of infection and fever, leucocytosis, tachypnea, and tachycardia explained by presence of different dermatoses known to be associated with such findings namely Hansen′ disease with type 2 reaction 32(50.79%) patients, pustular or extensive plaque psoriasis 13(20.64%), drug rash including SJS 5(7.94%), cutaneous lymphoma 5(7.94%), systemic lupus erythematosus 4(6.35%), pyrexia of unknown origin 4(6.35%) patients. All patients in dermatology ward were admitted with dermatological disorders , four patient developed pyrexia for which no cause could be found (PUO) but they fulfilled SIRS criteria. These patients did not have widespread erosions, blood cultures were negative and they were excluded from sepsis cases.
Profile of sepsis patients
A total of 40 patients (4.65%) of mean age 34.95 ± 15.13 years (range 4−62 years) developed sepsis. This included 23 women (57.5%) and 17 men (42.5%). Majority of them (67.5%) fulfilled all four SIRS features and 34 (85%) cases had documented bacteremia.
The 40 patients with sepsis included 17 (42%) with autoimmune vesicobullous diseases, 10 (25%) with erythroderma, 9 (22%) with SJS-TEN, and one each (3%) with drug-induced maculopapular rash, non-Hodgkin lymphoma, dermatomyositis, and dermatitis artefacta. The autoimmune vesicobullous diseases included pemphigus vulgaris 13 patients, pemphigus foliaceous in two patients and one each with paraneoplastic pemphigus and chronic bullous dermatosis of childhood.
The proportion of patients developing sepsis was highest in toxic epidermal necrolysis (TEN) (9/10; 90%), followed by drug-induced maculopapular rash (1/5; 20.0%), erythroderma (10/57; 17.5%), and vesicobullous diseases (17/200; 8.5%). The mean duration of hospitalization in sepsis cases was 39.5 ± 26.3 days which was significantly more than that of all admitted patients (19.25 ± 15.65 days, P value < 0.05).
Nosocomial and community-acquired sepsis
Fifteen patients (37.5%) had community-acquired sepsis while 25 (62.5%) had onset of sepsis after 48 h of hospitalization. Nine (60%) community-acquired sepsis patients died as compared to six (24%) of nosocomial sepsis (P = 0.023).
Predisposing factors for sepsis
Common (known) risk factors which contribute to development or perpetuation of sepsis as mentioned in various other studies were use of immunosuppressive agents (31 patients; 77.5%), recent hospitalization (10; 25.0%), diabetes mellitus (5; 12.5%), and smoking (5; 12.5%). The body surface area (BSA) involvement was also extensive (mean 53.6% ± 34.1%) in sepsis cases.
Systemic involvement in sepsis patients
Other systems involved in sepsis patients were respiratory system, with pneumonitis occurring in 17 (42.5%), pleural effusion in 5 (20.0%), and aspiration pneumonitis in 1 (2.5%) patient, cardiovascular system in 6 (15.0%), and central nervous system involvement in 7 (17.5%) cases. These were supported by various laboratory investigations.
Severe sepsis and death
Seventeen of 40 (42.5%) sepsis patients deteriorated to develop features of severe sepsis. Following features of advanced sepsis were present, hypoxemia 15 (37.5%), hypotension 12 (30.0%), metabolic acidosis 9 (22.5%), oliguria 9 (22.5%), acute encephalopathy 5 (12.5%), and DIC 5(12.5%).
Fifteen (37.5%) sepsis patients died. Seven of 17 (41.2%) pemphigus patients with sepsis died, while three (3/9; 30.0%) each of erythroderma and TEN succumbed to sepsis. There were total 10 patients admitted with TEN, out of which 9 developed sepsis; of which 3 (33.3%) died due to sepsis. One patient each of drug induced maculopapular rash and dermatomyositis also died due to sepsis. Patient had widespread maculopaular rash at admission and became generalized and patient had jaundice. Patient received corticosteroids for drug hypersensitivity syndrome. Death was due multiple factors including infection and liver failure.
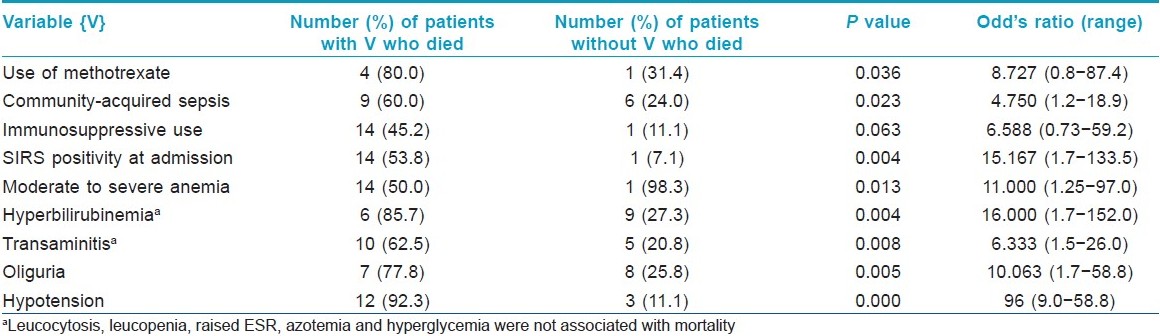
Total number of deaths in the ward during the study period due to all causes were 22; thus sepsis contributed to 68.2% cases of death in the ward. Important risk factors assessed for death in sepsis on clinical evaluation were age, sex, duration of dermatoses, time between admission and sepsis, fever at admission, presence of cough, burning micturition, chronic smoking and alcohol use, hypertension, diabetes mellitus, concomitant chronic illness, immunosppressive drug intake and number of pulse therapies and only concomitant chronic illness, methotrexate use, and duration of hospitalization were found to significantly affect outcome. In addition, many parameters indicative of severe sepsis like pneumonia, hypoalbuminemia, organ involvement as renal failure, encephalopathy, hypotension were associated with poor outcome. Approximately 50 variables were correlated with death in sepsis and only those statistically significantly associated with death are listed in [Table - 1]. Hyperbilirubinemia and transaminitis were part of drug reactions or advanced sepsis and not due to methotrexate. The body surface area involvement (P = 0.423), isolation of MRSA (P = 0.987), acinetobacter (P = 0.227), pseudomonas (P = 0.768), klebsiella (P = 0.579) were not associated with increased deaths in sepsis patients.
Culture characteristics in sepsis patients
Patients with sepsis (N = 40) were admitted for a long time (average 39.5 ±26.3 days) and were subjected to microbiological investigations till they had features of sepsis, thus leading to multiple isolates. (A total of 382 bacterial isolates were obtained from 956 specimens).
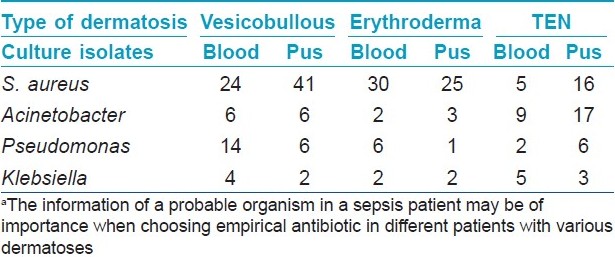
Methicillin-resistant Staphylococcus aureus (MRSA) was the commonest organism isolated (99; 25.9%) in all culture specimens followed by Acinetobacter spp. (52; 13.6%), Pseudomonas spp. (40; 10.5%), and others [Table - 2]. In vesicobullous disorders or erythroderma, staphylococcus was the predominant isolate in blood, while in TEN, Acinetobacter spp. outnumbered others [Table - 3]. MRSA was mostly nosocomial and out of 99 isolates only 8 were community acquired.
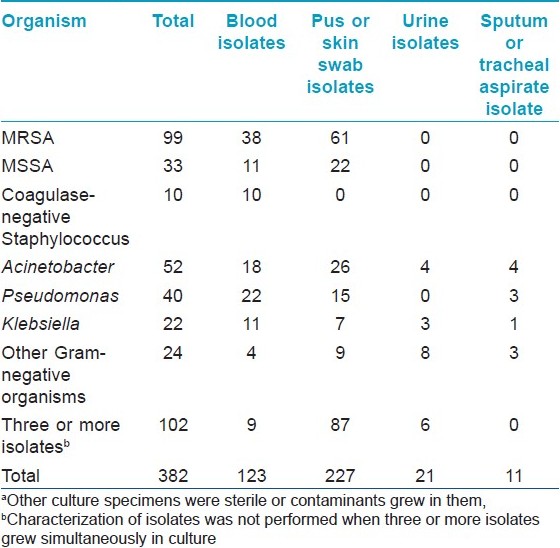
Different patients had variable infections during their hospital stay. MRSA was isolated from 24 patients, MSSA in 19, acinetobacter in 16 patients, pseudomonas in 13 patients, and klebsiella in 9 patients at different points of time. The source of bacteremia (concordance between blood and other culture isolates) could be found in majority of the patients (23/40). Cutaneous infection was the commonest source of bacteremia (20 patients). The type of organism causing sepsis had no statistically significant impact on the outcome (mortality).
Sensitivity patterns
MRSA showed 100% sensitivity to vancomycin, teicoplanin followed by linezolid (77/78; 98.7%), rifampicin (70/85; 82.4%), netilmycin (53/82; 64.6%), clindamycin (15/32; 45.5%), amikacin (35/88;39.8%), cotrimoxazole (18/86;20.9%), erythromycin (14/83; 16.9%), ciproflox (13/89; 14.6%), penicillin (2/66; 0.03%), cloxacillin (0/64; 0.0%). MSSA showed 100% sensitivity to vancomycin, teicoplanin, linezolid, and netilmycin followed by amikacin (22/24; 94.1%), rifampicin (22/25; 88.0%), erythromycin (13/22; 59.1%), penicillin (14/24; 58.3%), clindamycin (4/7; 57.1%), cotrimoxazole (12/25; 50.0%), and ciprofloxacin (9/23; 39.1%).
Acinetobacter was mostly sensitive (48/53; 90.6%) to cefoperazone and sulbactum combination followed by imepenem (26/48; 54.2%), piperacillin + tazobactum (25/50; 50%), meropenem (19/99; 38.8%), netilmycin (17/47; 36.2%), ticaricillin + clavulinic acid (9/45; 20.0%), and 15−20% were sensitive to piperacillin and amikacin (10/52, 19.2%) and 4% to cefazidime and ciproflox.
Pseudomonas was sensitive to cefoperazone + sulbactum (31/39; 79.5%), piperacilin + tazobactum (31/40; 77.5%), imepenem (20/32; 62.5%), piperacillin (19/33; 54.3%), ticarcillin + clavulinic acid (19/39; 48.7%), meropenem (17/36; 47.2%), amikacin (16/38; 42.1%), ciprofloxacin (15/37; 40.5%), netilmycin (9/38; 23.7%), ceftazidime(2/38; 5.3%).
Klebsiella species were sensitive to cefoperazone + sulbactum (27/28; 96.4%), meropenem (18/24; 75.0%), imepenem (15/23; 65.2%), piperacillin + tazobactum (15/24; 64.3%), amikacin (10/27; 37.4%), ticarcillin + clavulinic acid (7/23; 30.4%), netilmycin (7/27; 26.9%), ciproflox (4/28; 14.3%), and ceftazidime (2/27; 7.4%).
Combined sensitivity for gram-negative organisms isolated was cefoperazone + sulbactum (125/139; 89.9%), piperacilin + tazobactum (93/128;72.7%), imepenem (71/123; 62.8%), meropenem (72/129; 55.8%).
Discussion
There is a lack of consensus regarding a clear definition of sepsis. SIRS criteria alone has low sensitivity and specificity. SIRS criteria alone were not adequate for sepsis diagnosis in dermatology inpatients in our study. It was essential to add strongly suspected or documented infection to increase the specificity of the sepsis definition. In our study, 63 false-positive SIRS positive patients were excluded as there was valid explanation for symptoms and signs included in SIRS. We propose from our experience that SIRS criteria may be used for only screening in dermatology wards and sepsis to be suspected, if the patient has widespread skin erosions and is not able to get up from bed and loss of eye contact with physician during the rounds. There is an urgent need to develop criteria for sepsis in dermatology inpatients.
The comparison between different studies pertaining to sepsis appears to be complicated because of the nonhomogenous nature of the patient populations studied and of methods adopted by various authors. [11]
The incidence of sepsis in our dermatology in-patients was 4.65%, whereas the reported incidence of bacteremia varied from 2.5% to 18% cases in dermatology wards. [12],[13],[14],[15]
We compared the incidence of sepsis in our TEN patients to other studies involving TEN or burn patients as both have extensive denudation of skin. The incidence of sepsis in our TEN patients was 90%, higher than reported in other studies (41.7%−83.3%) or in burn patients (28%−55%); [2],[13],[16],[17],[18],[19] however, our study included only 10 patients with TEN.
In our ward, sepsis contributed to 68.2% of all deaths during the study period, while in another study, it contributed to 10.8% deaths. [3] Sepsis associated high mortality rates have been reported from various burn units (29.1%−71.4%), surgical wards and ICUs (30%−50%) both in India and abroad. [5],[6],[18],[20],[21],[22] This is comparable to that observed in our patients (37.5%). Death rates of 20%−44% have been reported in TEN patients managed at burn wards which are comparable to mortality in our TEN patients with sepsis (33.3%). [23],[24] We analyzed correlation with nearly 50 variables for death in sepsis cases but found statistically significantly association with intake of methotrexate, community acquired sepsis, SIRS positivity at admission, hypotension, and laboratory investigations like moderate to severe anemia, hypoalbuminemia, hyperbilirubinemia, and transaminitis. Surprisingly prior intake of corticosteroids and azathioprine and pulse steroids did not significantly increase death rate in sepsis (P = 0.063). The high death rate in community acquired sepsis and SIRS positivity was probably due to several patients being brought to our hospital in terminal stage of disease. The high rate of death in patients receiving methotrexate was surprising and needs to be confirmed in larger studies. It seems in our study that concomitant liver damage was associated with higher mortality.
Expectedly, infection developing in cutaneous erosions or diseased skin was the most frequent source of infection in our patients (37 patients; 92.5%), this is supported by various studies, which have shown that wound and blood stream infections are more common in burn ward patients in contrast to surgical, medical or other ICU units where pneumonia and UTI are commoner. [6],[14],[18],[20],[22],[25] In our study, sepsis cases needed hospitalization for approximately 20 days more than nonsepsis patients which is comparable to the extra hospital stay period of 6−14 days reported from ICU and surgery wards. [16]
Different studies have reported different microbiological profile in sepsis patients. In a study of bacteremia episodes in 657 patients from various medical and surgical wards of our institute, S. aureus was the most common single isolate (29%), while overall, gram-negative bacteremia accounted for majority (60%) of the isolates. [26] Pseudomonas aeruginosa (31%−54%), S. aureus (22%−31%), and Klebsiella sp. (19-35%) have been implicated in different burn wards. [22],[27] In ICUs and other ward settings, coagulase negative Staphylococci (27%), S. aureus (15%), and enterococci (10%) have been shown to be the leading pathogens. [20],[21] In our study, S. aureus (MRSA > MSSA) was the leading pathogen followed by gram-negative organisms (acinetobacter > pseudomonas > klebsiella). These results are broadly similar to those isolations from ICU and surgical wards. In our study, there were some differences in isolates from different dermatoses and acinetobacter was isolated more frequently from TEN cases compared to other dermatoses [Table - 3].
In our study, the overall sensitivity patterns are comparable to the previous study conducted in the department of microbiology of our institute, in which S. aureus showed high sensitivity to vancomycin (100%), rifampicin (81.21%), and poor sensitivity to ciprofloxacin (52%) and ampicillin (49%). [28] Imepenem (62.8%) and meropenem (55.8%) also showed good sensitivity toward these isolates, while gram-negative organisms showed good sensitivity to piperacillin + tazobactum (94.4%). [28] It may be due to recent introduction of these agents in our hospital and their sparing use. Worldwide literature supports the high efficacy of newer antibiotics. [29] Studies from other hospitals in India including Delhi reflect relatively similar sensitivity profile as seen in our study. [30]
To conclude, the incidence of sepsis in the dermatology ward was 4.65%. Sepsis occurred most frequently in TEN cases. Fifteen of 40 sepsis patients died, the mortality rate being 37.5%. MRSA and gram-negative organisms were the predominant micro-organisms causing sepsis. Empirical antibiotic guidelines covering these bacteria could be planned for dermatology in-patients.
Although unique in its own, our study did not have controls and was limited to a dermatology ward in a tertiary care hospital. Results from this study should be carefully interpreted keeping individual population related demographic and microbiological variations in mind.
| 1. |
Ducic I, Shalom A, Rising W, Nagamoto K, Munster A. Outcome of patients with toxic epidermal necrolysis syndrome revisited. Plast Reconstr Surg 2002;110:768-73.
[Google Scholar]
|
| 2. |
Mahajan VK, Sharma NL, Sharma RC, Garg G. Twelve-year clinico-therapeutic experience in pemphigus: A retrospective study of 54 cases. Int J Dermatol 2005;44:821-7.
[Google Scholar]
|
| 3. |
Nair PS, Moorthy PK, Yogiragan K. A study of mortality in dermatology. Indian J Dermatol Venereol Leprol 2005;71:23-5.
[Google Scholar]
|
| 4. |
Kanwar AJ, Dhar S. Factors responsible for death in patients with pemphigus. J Dermatol 1994;21:655-9.
[Google Scholar]
|
| 5. |
Pittet D, Tarara D, Richard P. Nosocomial bloodstream infection in critically ill patients. J Am Med Assoc 1994;271:1598-601.
[Google Scholar]
|
| 6. |
Orgeas MG, Chevret S, Mainardi JL, Timsit JF, Misset B, Carlet J. A one-year prospective study of nosocornial bacteremia in ICU and non-ICU patients and its impact on patient outcome. J Hosp Infect 2000;44:206-13.
[Google Scholar]
|
| 7. |
Askarian M, Gooran NR. National nosocomial infection surveillance system-based study in Iran: Additional hospital stay attributable to nosocomial infections. Am J Infect Control 2003;31:465-8.
[Google Scholar]
|
| 8. |
Bone RC, Balk RA, Cerra FB, Dellinger RP, Fein AM, Knaus WA, et al. American College of chest physician / society of critical care medicine consensus conference, definition for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. Crit Care Med 1992;20:864-74.
[Google Scholar]
|
| 9. |
Collec JG, Miles RS, Wan B. Tests for the identification of bacteria. In: Mackie and McCarteny, editors. Practical medical microbiology, 14 th ed. Edinburg: Churchill Livingstone; 1996. p. 131-50.
[Google Scholar]
|
| 10. |
Wayne PA. Performance standards for antimicrobial disk susceptibility testing. National Comm Dis 2002;12:130-40.
[Google Scholar]
|
| 11. |
Riedmann NC, Guo RF, Ward PA. The enigma of sepsis. J Clin Investig 2003;112:460-7.
[Google Scholar]
|
| 12. |
Askarian M, Gooran NR. National nosocomial infection surveillance system-based study in Iran: Additional hospital stay attributable to nosocomial infections. Am J Infect Control 2003;31:465-8.
[Google Scholar]
|
| 13. |
McManus AT, Mason AD Jr, McManus WF, Pruitt BA Jr. A decade of reduced gram-negative infections and mortality associated with improved isolation of burned patients. Arch Surg 1994;129:1306-9.
[Google Scholar]
|
| 14. |
Zhang Y. A two-year prospective survey on nosocomial infections. Zhonghua Yi Xue Za Zhi 1991;71:253-6.
[Google Scholar]
|
| 15. |
Dettenkofer M, Wilson C, Ebner W, Norgauer J, Ruden H, Daschner FD. Surveillance of nosocomial infections in dermatology patients in a German university hospital. Br J Dermatol 2003;149:620-3.
[Google Scholar]
|
| 16. |
Ward DJ, Krzeminska EC, Tanner NS. Treatment of toxic epidermal necrolysis and a review of six cases. Burns 1990;16:97-104.
[Google Scholar]
|
| 17. |
Brand R, Rohr JB. Toxic epidermal necrolysis in Western Australia. Australas J Dermatol 2000;41:31-3.
[Google Scholar]
|
| 18. |
Santucci SG, Gobara S, Santos CR, Fontana C, Levin AS. Infections in a burn intensive care unit: Experience of seven years. J Hospital Inf 2003;53:6-13.
[Google Scholar]
|
| 19. |
B���ang RL, Gang RK, Sanyal SC, Mokaddas E, Ebrahim MK. Burn septicemia: An analysis of 79 patients. Burns 1998;24:354-61.
[Google Scholar]
|
| 20. |
Lark RL, Chenoweth C, Saint S, Zemencuk JK, Lipsky BA, Plorde JJ. Four-year prospective evaluation of nosocomial bactermia: Epidermiology, microbiology and outcome. Diagn Microbiol Infect Dis 2000;38:130-40.
[Google Scholar]
|
| 21. |
Valles J, Leon C, Lerma FA. Nosocomial bacteremia in critically ill patients: A multicenter study evaluating epidemiology and prognosis. Clin Infect Dis 1997;4:387-95.
[Google Scholar]
|
| 22. |
Taneja N, Emmanuel R, Chari PS, Sharma M. A Prospective study of hospital-acquired infections in burn patients at a tertiary care referral center in north India. Burns 2004;30:665-9.
[Google Scholar]
|
| 23. |
Schulz JT, Sheridan RL, Ryan CM, MacKool B, Tompkins RG. A 10-year experience with toxic epidermal necrolysis. J Burn Care Rehabil 2000;21:199-204.
[Google Scholar]
|
| 24. |
Wong KC, Kennedy PJ, Lee S. Clinical manifestations and outcomes in 17 cases of Stevens-Johnson syndrome and toxic epidermal necrolysis. Australas J Dermatol 1999;40:131-4.
[Google Scholar]
|
| 25. |
Duggan J, O'Connell D, Heller R, Ghosh H. Causes of hospital acquired septicemia. Q J Med 1993;86:479-48.
[Google Scholar]
|
| 26. |
Mathur P, Kapil A, Das B, Dhawan B. Epidemiology and microbiology of nosocomial bacteremia at an Indian tertiary care hospital. Trop Doct 2005;35:167-8.
[Google Scholar]
|
| 27. |
Singh NP, Goyal R, Manchanda V, Das S, Kaur I, Talwar V. Changing trends in bacteriology of burns in burns unit, Delhi, India. Burns 2003;29:129-32.
[Google Scholar]
|
| 28. |
Mohanty S, Kapil A, Dhawan B, Das BK. Bacteriological and antimicrobial susceptibility profile of soft tissue infections from northern India. Indian J Med Sci 2004;58:10-5.
[Google Scholar]
|
| 29. |
Turner PJ. Trends in antimicrobial susceptibilities among bacterial pathogens isolated from patients hospitalized in European medical centers: 6-year report of the MYSTIC Surveillance Study (1997-2002). Diagn Microbiol Infect Dis 2005;51:281-9.
[Google Scholar]
|
| 30. |
Agnihotri N, Gupta V, Joshi RM. Aerobic bacterial isolates from burn wound infections and their antibiograms: A five-year study. Burns 2004;30:241-3.
[Google Scholar]
|
Fulltext Views
3,749
PDF downloads
4,958





