Translate this page into:
Clinical and demographic characteristics of mucous membrane pemphigoid in India: A retrospective analysis
Corresponding author: Prof. Sanjeev Handa, Department of Dermatology, Venereology and Leprology, Postgraduate Institute of Medical Education and Research, Chandigarh, India. handa_sanjeev@yahoo.com
-
Received: ,
Accepted: ,
How to cite this article: De D, Hanumanthu V, Jinagal J, Handa S, Mahajan R, Chatterjee D, et al. Clinical and demographic characteristics of mucous membrane pemphigoid in India: A retrospective analysis. Indian J Dermatol Venereol Leprol. 2024;90:763-8. doi: 10.25259/IJDVL_273_2023
Abstract
Background
Mucous membrane pemphigoid (MMP) is a rare subepidermal autoimmune blistering disorder. The clinical and demographic parameters of this disease in Indian patients have not yet been elucidated in detail.
Objective
We aimed to study the clinical and demographic characteristics, disease course, and treatment aspects of MMP patients.
Methods
The data for this study were obtained by reviewing the case record forms of patients registered in the Autoimmune Bullous Disease (AIBD) Clinic of the Department of Dermatology, Venereology & Leprology, Postgraduate Institute of Medical Education and Research, Chandigarh, a tertiary care centre in India. The diagnosis of MMP was established on the basis of clinical and immune-histopathological features which are consistent with standard diagnostic criteria for the disease.
Results
A total of 52 patients with MMP registered in the AIBD clinic were included. The mean age at disease onset was 50 years and the average age at presentation was 56 years. Females outnumbered males in the study with a ratio of 1.36:1.
The oral and ocular mucosae were the most commonly affected sites (82.6% and 63.4% respectively). Visual difficulty was reported by half the patients (26 of 52 patients).
IgG, C3, and IgA deposits were detected on direct immunofluorescence (DIF) in 29, 21, and 11 patients, respectively. Serologic analysis was performed in only 7 of the patients and of these, just 1 exhibited a positive result on multivariant ELISA and epidermal pattern of binding on salt split skin indirect immunofluorescence.
Most patients were treated with prednisolone (44 of 52). Steroid-sparing adjuvants were used in combination including cyclophosphamide, azathioprine, methotrexate, dapsone, and colchicine. Rituximab was administered in 7 patients with severe or refractory disease.
Limitations
This is a retrospective analysis of data available from a clinic registry. In patients with negative direct immunofluorescence on biopsy, the diagnosis was based on clinico-pathologic consensus.
Conclusion
MMP is not as uncommon in India as the paucity of reports suggest. Visual complications are frequent in Indian MMP patients. A high index of suspicion is required for early diagnosis and appropriate treatment to prevent ocular complications.
Keywords
Mucous membrane pemphigoid
autoimmune bullous disease
histopathology
direct immunofluorescence
treatment
India
Introduction
Mucous membrane pemphigoid (MMP) is a chronic, subepithelial autoimmune bullous disease (AIBD) predominantly affecting the mucosal surfaces. The lesions tend to heal with scarring.1 It frequently affects the oral mucosa and conjunctiva, but the skin and other mucosae such as the nasopharynx, anogenital mucosa, larynx, and esophagus may also be involved.2 It is seen more frequently in women and in individuals in their fifth and sixth decades of life.3–5 Ocular cicatricial pemphigoid (OCP) is a variant of MMP that involves the ocular mucosa alone leading to conjunctival scar formation and serious sequelae.6
The pathogenesis of MMP is unclear. Autoantibodies (IgG, IgA or both) targeting six molecular antigens in the hemidesmosomes and lamina lucida have been demonstrated.2 This results in disruption of the junction between epithelial and subepithelial zones, causing blister formation. The lesions often heal with scarring, sometimes leading to devastating complications such as breathing difficulty, dysphagia, and blindness with a significant impact on the quality of life.
We could identify only 2 earlier reports (both from our institution) describing the clinical and demographic characteristic of MMP in India.7,8 This study presents a retrospective analysis of the clinico-demographic parameters of MMP in India.
Patients and Methods
This is a retrospective analysis of data collected from the case record forms of MMP patients registered in the Autoimmune Bullous Disease (AIBD) Clinic of the Department of Dermatology, Venereology & Leprology, Postgraduate Institute of Medical Education and Research, Chandigarh, a tertiary care centre in India from November 2013 to March 2022. Institute Ethics Committee approval was obtained (IEC-INT/2023/Study-864).
The diagnosis of MMP was essentially clinical, based on the presence of chronic, inflammatory, or blistering disease with scarring affecting the mucous membranes, sometimes with skin involvement. In this clinical context, the presence of linear deposition of IgG, IgA, or both and complement proteins (C3) along the subepithelial basement membrane zone in direct immunofluorescence (DIF) was confirmatory, but not essential for this study. In patients with a negative DIF, the diagnosis was reached by consensus after discussion between the clinicians (dermatologists and ophthalmologists) and pathologists based on the clinical presentation, histopathological assessment, and correlation with other diagnostic tests such as serology (when available). Indirect immunofluorescence (IIF) and enzyme-linked immunosorbent assay (ELISA) were not available for most of the study duration and immunoblot is still unavailable.
Ocular cicatricial pemphigoid (OCP) accounts for about 2/3 of all MMP cases.9,10 A negative DIF may be seen in almost half of these cases regardless of the biopsy site. Thus, the diagnosis of OCP was made by excluding other causes of cicatrising conjunctivitis like Stevens–Johnson syndrome, toxic epidermal necrolysis, chronic pilocarpine, or other anti-glaucoma medication use, Sjogren syndrome, ocular rosacea, severe atopic kerato-conjunctivitis, chemical injury, and chronic graft versus host disease.
Study population, data collection, and statistical analysis
The clinical characteristics of the 52 MMP patients were extracted. These included age, gender, duration of the disease, location of lesions, histopathologic and DIF findings, treatment received, response to treatment, complications, and sequelae.
Disease control (DC) was defined as the time at which new inflammatory lesions ceased to form and healing of already established lesions began. Clinical remission (CR) was defined as the absence of new or already established lesions while the patient was off therapy for at least 2 months.11
Serological data pertaining to indirect immunofluorescence (IIF) on salt-split skin and commercially available multivariant ELISA (Euroimmun, Luebeck, Germany) were available from October 2021. The slat split skin IIF was considered positive when there was staining of IgG and/or IgA on the epidermal side with or without dermal side staining.12
The data were analysed using the statistical package for social services (SPSS version 20 SPSS Inc., Chicago. IL). For descriptive analysis, continuous variables such as age of onset, total duration of illness, age at presentation, and time to remission are reported as means and standard deviations (SD) (median and range for skewed data). Categorical variables are reported as percentages and proportions.
Results
The details regarding the clinico-demographic profile of MMP are summarised in Table 1.
| Characteristics of MMP (n = 52) | |
| Age at presentation (years) | 56 ± 15.15 (Mean ± SD) |
| Age at onset (years) | 50.65 ± 16.14 (Mean ± SD) |
| Sex (male: female) | 1: 1.36 |
| Total duration of illness in months, median (range) | 26.5 (0.5 to 360) |
| Site of involvement | |
| Oral mucosa | 43 (82.6%) |
| Oral mucosa alone (out of 43) | 14 (32.5%) |
| Ocular mucosa | 33 (63.4%) |
| Ocular mucosa alone (out of 33) | 7 (21.2%) |
| Nasal mucosa | 12 (23%) |
| Anogenital mucosa | 11 (21%) |
| Cutaneous involvement | 12 (23%) |
| Triggering factor | |
| Cataract surgery | 3 |
| Autoimmune associations | |
| Rheumatoid arthritis | 3 |
| Type 1 diabetes mellitus | 1 |
| Hypothyroidism | 1 |
| Complications associated with other mucosal involvement | |
| Decrease in vision (out of 33) | 26 (78.8%) |
| Dysphagia | 2 |
| Change in voice | 3 |
MMP- Mucous membrane pemphigoid, SD- Standard deviation
The gingivobuccal sulcus was the most common oral mucosal site involved, followed by the hard palate and tongue [Figure 1a–1d]. Ocular mucosal involvement (conjunctival congestion, scanty serous or mucoid discharge, photophobia, decreased visual acuity, corneal clouding, synechiae/symblepharon, shortening of fornices, ectropion, and trichiasis) was observed in 33 patients [Figure 2a–2d]. Ocular involvement alone (i.e., OCP) was noted in 7 patients.
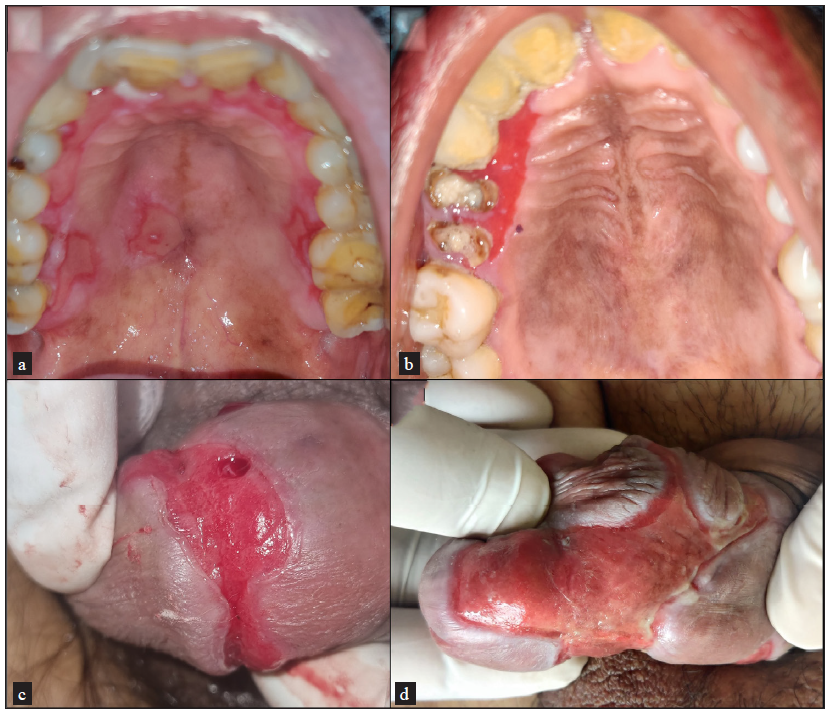
- Clinical pictures of mucous membrane pemphigoid patients showing (a) involvement of hard palate, (b) gingival mucosa, and (c and d) penile region with erythematous erosion and lichenoid hue at the periphery.
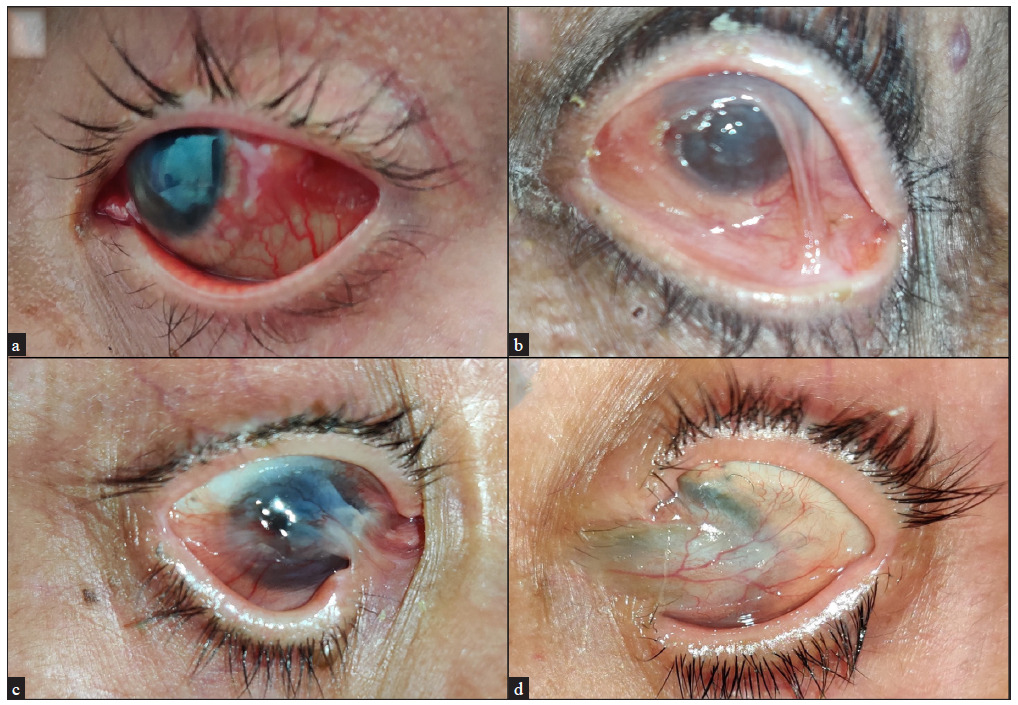
- Clinical pictures of ocular mucous membrane pemphigoid patients showing (a) conjunctival congestion, (b) synechiae, (c) symblepharon and shortening of fornices, and (d) corneal clouding
Complications were observed in 29 patients. Gingivobuccal adhesions were noted in 2 of the 43 (4%) patients with oral involvement and ocular adhesions (synechiae /symblepharon) in 27 of the 33 patients with eye involvement.
Most biopsies (43 cases) were taken from oral mucosa for histopathology and DIF. Oral biopsies revealed a subepithelial split in 8 cases with infiltration of eosinophils and neutrophils in 7 and 4 cases, respectively. Lymphomononuclear and plasma cells were noted in the lamina propria. Subepithelial fibrosis and scattered fibroblasts were notable in patients with scarring.
Conjunctival biopsies were performed on 9 patients with ocular involvement. Seven of these patients had ocular involvement alone. A positive DIF along with subepithelial cleft was observed in two patients. Additionally, other histopathological features such as subepithelial fibrosis and lympho-plasmocytic infiltrate with or without cleft were also detected in the patients who underwent conjunctival biopsy.
DIF revealed IgG deposits in 56% (29/52) of patients. C3 deposits and IgA in a linear pattern along the epithelial–subepithelial junction were noted less frequently (40% and 21% of the patients, respectively). Only one of the 7 patients in whom serologic analysis with multivariant ELISA was performed was seropositive to BP180 and had IgG staining in the epidermal side on human salt split skin IIF [Figure 3a–3d].
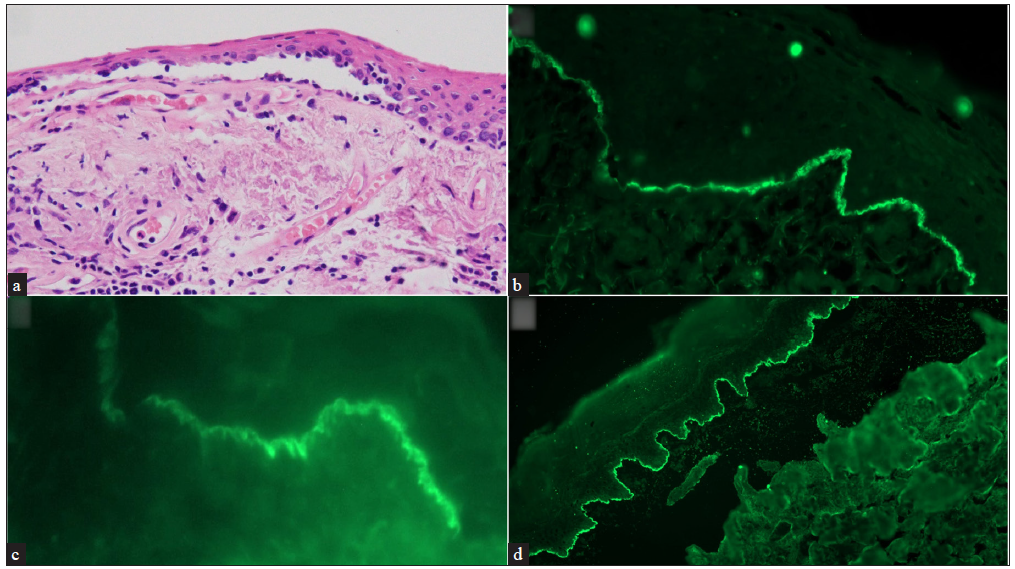
- (a) Conjunctival biopsy showing a sub-epithelial cleft. The sub-epithelium showsfibrosis with lymphomononuclear infiltrate (hematoxylin and eosin, 200x), (b) Direct immunofluorescence (DIF) showing strong linear IgG deposits along the epithelial basement membrane zone (Fluorescein isothiocyanate, 400x), (c) High power of DIF showing “n” serration pattern (Fluorescein isothiocyanate, 630x), and (d) salt split skin indirect immunofluorescence showing roof pattern binding for IgG (Fluorescein isothiocyanate, 200x)
Three of the 52 patients were lost to follow-up after initiation of oral prednisolone. Thus, the response to therapy was available in only 49 of the 52 patients. Oral prednisolone at a dose of 0.5 mg/kg body weight was instituted in 44 of these 49 patients (89.7%). Topical triamcinolone acetonide application was advised for oral erosions. Systemic therapies (cyclophosphamide, dapsone, azathioprine, rituximab, methotrexate, and colchicine) were initiated either alone or in combination with prednisolone. CR was achieved in 14 patients (27%) within a mean period of 4.85 ± 5.3 months (mean ± SD). Twelve of these 14 patients were treated concurrently with cyclophosphamide as an adjuvant. In seven patients with severe disease (i.e., multisite involvement) or treatment failure, rituximab was administered to achieve DC.
We observed 5 adverse drug events affecting 4 (8%) of the 49 patients. These included 3 instances of cyclophosphamide induced lymphopenia, and a single case each of dapsone hypersensitivity syndrome and azathioprine induced transaminitis [Table 2].
| Characteristics of MMP patients | Results |
|---|---|
| Direct immunofluorescence positive with any one or more of the following immunoreactants | 30 (57.6%) |
| IgG | 29 (55.7%) |
| IgA | 11 (21.1%) |
| IgM | 4 (7.6%) |
| C3 | 21 (40.3%) |
| Profile ELISA (of 7 patients) | 1 (14.2%) |
| Indirect Immunofluorescence on human salt split skin (IIF-SSS) (of 7 patients) | 1 (14.2%) |
| Treatment received | 49 (94.2%) |
| Prednisolone alone | 5 (10.2%) |
| Cyclophosphamide ± prednisolone | 24 (48.9%) |
| Azathioprine ± prednisolone | 9 (18.3) |
| Dapsone ± prednisolone | 18 (36.7%) |
| Rituximab ± prednisolone | 7 (14.2) |
| Methotrexate ± prednisolone | 4 (8%) |
| Time to achieve clinical remission in months (of 14 patients) | 4.85 ± 5.3 months (mean ± SD) |
| Adverse drug events (total 5 events in 4 patients) | Cyclophosphamide- Leukopenia in 3 patients |
| Dapsone induced hypersensitivity- 1 patient | |
| Azathioprine - transaminitis in 1 patient | |
| Complications/sequelae of disease | 29 (55.7%) |
| Synechiae/symblepharon (Out of 33 with eye involvement) | 27 (81.8%) |
| Gingiva buccal adhesions (Out of 43 with oral mucosal involvement) | 2 (4.6%) |
#ELISA: - Enzyme linked immunosorbent assay; IIF-SSS- Indirect immunofluorescence salt split skin; MMP- Mucous membrane pemphigoid, SD- Standard deviation
Discussion
Data regarding the clinical and demographic characteristics of MMP patients in India are limited.2 Consistent with previous reports, we too observed presentation in the fifth and sixth decades (mean age 56 ± 15.15 years) and a female predominance (male: female – 1: 1.36).2
The interval between disease onset and presentation varied widely (15 days to 3 years; median 26.5 months). The delay in presentation is of concern and may have been due to the fact that ophthalmologists, otorhinolaryngologists, or dentists were often consulted first owing to the predominant mucosal involvement. Some patients with conjunctival involvement alone were referred to us from ophthalmology for confirmation of the diagnosis or to exclude the possibility of alternate diagnoses. Clinical features during the initial phase of ocular MMP are non-specific - watering of eyes, redness, photophobia, dryness, foreign body sensation, and irritation. This may later lead to conjunctival fibrosis, shortening of fornices, trichiasis, symblepharon, and synechiae with permanent visual disability.13 Prompt recognition and action are thus essential to avoid permanent disfigurement.6 Prior to any eye surgery (e.g. for cataract, etc), stabilisation of the ocular surface and correction of lid margin–related issues are mandatory in ocular MMP to avoid disease exacerbation, as was observed in three patients of our study.14
In our study, the oral mucosa was the most frequently affected site (82.6% of cases), which is similar to that reported in earlier studies.15 Oral mucosal involvement has been reported to occur in 85% of MMP patients with the gingivae (80%) being most commonly affected, while the labial mucosa remains uninvolved.5,16,17 Isolated desquamative gingivitis with non-specific histopathological changes and DIF positivity was observed in a single patient in our study. While it can be challenging to distinguish MMP clinically from erosive lichen planus when it appears as isolated desquamative gingivitis, the lack of peripheral Wickham’s striae along with the histopathological findings and a positive DIF aid in differentiating these diseases.18, 19 Visible scar formation in the oral mucosa is unusual,20 but subtle scarring and fibrosis may sometimes be appreciated by rolling the fingers on the buccal mucosa. Conjunctival scarring leading to a limitation of daily activities is a prominent feature of ocular lesions, and synechiae or symblepharon were noted in 82% of our patients with eye involvement.
Malik et al. reported the association of autoimmune connective tissue disorders such as systemic lupus erythematosus (SLE) and mixed connective diseases with MMP.21 Although we did not carry out active screening for co-existing autoimmune disease or malignancy, 3 patients in our study had rheumatoid arthritis and one each had type 1 diabetes mellitus and hypothyroidism, which corresponds to the findings from a US-based registry.4
Predominant mucosal involvement, scarring, and positive DIF with linear deposits of immune reactants at the basement membrane zone confirm the diagnosis of MMP.2 IgG was the commonest immune reactant noted in our study followed by IgA and C3, as observed in previous studies.16 Serologic analysis had limited diagnostic value compared to DIF with only one out of seven patients testing positive.12 Although 22 patients had a negative DIF, MMP was suspected based on distinct signs of ocular involvement after excluding other secondary causes of conjunctival scarring. A plausible explanation for the negative DIF could be disease quiescence or burnout.9
Most patients were treated with prednisolone (44 of 52). Steroid-sparing adjuvants were used in combination with prednisolone. These included cyclophosphamide in 24 patients, dapsone in 18, azathioprine in 9, rituximab in 7, methotrexate in 4, and colchicine in 2 patients.
High-risk group, i.e., extraoral mucosal involvement necessitates management with adjuvants like cyclophosphamide, methotrexate and azathioprine with oral steroids. In refractory or severe disease, rituximab infusions were administered as per rheumatoid arthritis protocol (two intravenous infusions of 1000 mg rituximab on days 1 and 15).
A multidisciplinary approach involving dermatologists, ophthalmologists, otorhinolaryngologists, gynaecologists, and other specialities wherever necessary is essential for the optimal management of MMP. As remissions and exacerbations are commonly seen in MMP, long-term follow-up is mandatory to prevent scarring and permanent disfigurement as well as to reduce morbidity.
Limitations
This is a retrospective analysis of data available from the clinic registry. Quality of life assessment was not planned. In patients with negative DIF, the diagnosis was based on clinico-pathologic consensus.
Conclusion
As the initial presentation of MMP is often non-specific and diagnostic modalities may have low sensitivity, a high index of suspicion is required for early diagnosis and prevention of potential development of permanent disability, especially related to ocular complications. A larger prospective study is needed to understand the exact burden of the disease and clinico-demographic characteristics of MMP patients in India.
Ethical approval
The Institute Ethics Committee approval was obtained (IEC-INT/2023/Study-864).
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
References
- European guidelines (S3) on diagnosis and management of mucous membrane pemphigoid, initiated by the European Academy of Dermatology and Venereology - Part I. J Eur Acad Dermatol Venereol. 2021;35:1750-64.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- World workshop on oral medicine VI: A systematic review of the treatment of mucous membrane pemphigoid. Oral Surg Oral Med Oral Pathol Oral Radiol. 2015;120:161-71. e20
- [CrossRef] [PubMed] [Google Scholar]
- A retrospective study of patient-reported data of bullous pemphigoid and mucous membrane pemphigoid from a US-based registry. Front immunol. 2019;10:2219.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Mucous membrane pemphigoid and oral blistering diseases. Clin Exp Dermatol. 2019;44:732-9.
- [CrossRef] [PubMed] [Google Scholar]
- Ocular mucous membrane pemphigoid: A review. Immunol Res. 2019;67:280-9.
- [CrossRef] [PubMed] [Google Scholar]
- Clinical and immunological study of mucous membrane pemphigoid in a cohort of Indian patients. Int J Dermatol. 2016;55:e557-61.
- [CrossRef] [PubMed] [Google Scholar]
- Clinical, demographic and immunopathological spectrum of subepidermal autoimmune bullous diseases at a tertiary center: A 1-year audit. Indian J Dermatol Venereol Leprol. 2016;82:358.
- [CrossRef] [PubMed] [Google Scholar]
- Clinical implications of direct immunofluorescence findings in patients with ocular mucous membrane pemphigoid. Am J Ophthalmol. 2017;183:48-55.
- [CrossRef] [PubMed] [Google Scholar]
- Ocular mucous membrane pemphigoid: Diagnosis and management strategies. Ocul Surf. 2008;6:128-42.
- [CrossRef] [PubMed] [Google Scholar]
- Definitions and outcome measures for mucous membrane pemphigoid: Recommendations of an international panel of experts. J Am Acad Dermatol. 2015;72:168-74.
- [CrossRef] [PubMed] [Google Scholar]
- Assessment of diagnostic strategy for mucous membrane pemphigoid. JAMA Dermatol. 2021;157:780-7.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Ocular pemphigoid. In: StatPearls. Treasure Island (FL): StatPearls Publishing; 2023.
- [Google Scholar]
- Cataract surgery in ocular surface diseases: Clinical challenges and outcomes. Curr Opin Ophthalmol. 2018;29:81-7.
- [CrossRef] [PubMed] [Google Scholar]
- Insights into clinical and diagnostic findings as well as treatment responses in patients with mucous membrane pemphigoid: A retrospective cohort study. J Am Acad Dermatol. 2022;87:48-55.
- [CrossRef] [PubMed] [Google Scholar]
- Direct immunofluorescence using non-lesional buccal mucosa in mucous membrane pemphigoid. Front Med (Lausanne). 2018;5:20.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Oral mucous membrane pemphigoid in a group of Thai patients-A 15-year retrospective study. J Dent Sci. 2022;17:1009-17.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Desquamative gingivitis: Clinical findings and diseases. J Am Acad Dermatol. 2018;78:839-48.
- [CrossRef] [PubMed] [Google Scholar]
- Desquamative gingivitis: Diagnosis and treatment. J Am Acad Dermatol. 2018;78:851-61.
- [CrossRef] [PubMed] [Google Scholar]
- World Workshop of Oral Medicine VII: A systematic review of immunobiologic therapy for oral manifestations of pemphigoid and pemphigus. Oral Dis. 2019;25(Suppl 1):111-21.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Coexistence of mucous membrane pemphigoid and connective-tissue disease. Clin Exp Dermatol. 2010;35:156-9.
- [CrossRef] [PubMed] [Google Scholar]






