Translate this page into:
Clinical efficacy of rituximab in the treatment of pemphigus: A retrospective study
Correspondence Address:
Vinod Kumar Sharma
Department of Dermatology and Venereology, All India Institute of Medical Sciences, New Delhi
India
| How to cite this article: Sharma VK, Bhari N, Gupta S, Sahni K, Khanna N, Ramam M, Sethuraman G. Clinical efficacy of rituximab in the treatment of pemphigus: A retrospective study. Indian J Dermatol Venereol Leprol 2016;82:389-394 |
Abstract
Background: Pulsed corticosteroids have been used successfully for the management of pemphigus. However, prolonged use of glucocorticoids may be associated with adverse effects and some patients show a poor response to conventional therapy. Biologics have shown a promising role in such cases; however, there is limited data from the Indian subcontinent. Objective: The primary objective was to assess the efficacy and adverse effects of rituximab in pemphigus. The secondary objective was to measure the cumulative doses of corticosteroids required for these patients. Methods: We undertook a retrospective review of records of 25 pemphigus patients (pemphigus vulgaris: 21, pemphigus foliaceus: 4) who had received rituximab infusion (rheumatoid arthritis protocol in 21 patients, modified in 4). Oral prednisolone was administered in dosages up to 0.5 mg/kg of body weight and tapered over the next 3–4 months according to the disease activity. However, other immunosuppressive agents such as cyclophosphamide and azathioprine were continued for one year after clinical remission was achieved. Results: Complete remission was observed in 22 (88%) patients. The mean time to disease control and complete remission was 1.10 and 4.36 months, respectively. Four (16%) patients experienced relapse after a mean duration of 11.75 months. The mean total dose of oral steroids administered was equivalent to 3535.64 mg of prednisolone. Exacerbation of disease was noted in two patients after the first dose of rituximab and infectious complications, pneumonia and cellulitis, developed in one patient each. Limitations: A small sample size, the retrospective nature of the study and unavailability of follow-up anti-desmoglein autoantibodies levels were limitations. Conclusion: Rituximab is an effective agent in the treatment of pemphigus. The use of rituximab enabled use of a lower initial dose of oral prednisolone in pemphigus and hence reduced its total cumulative dose. Severe side effects were rare.Introduction
Pemphigus is a group of rare, potentially fatal, autoimmune mucocutaneous blistering diseases with autoantibodies against epidermal adhesion proteins known as desmogleins. Pemphigus vulgaris is typically associated with autoantibodies to desmoglein 3 in mucosal-dominant disease and to desmoglein 3 and desmoglein 1 in mucocutaneous disease.[1]
Systemic glucocorticoids are still the most effective therapeutic agent for pemphigus.[2] Immunosuppressive agents such as cyclophosphamide, methotrexate, cyclosporine, mycophenolate mofetil and azathioprine are used in pemphigus for their steroid-sparing effect. Rituximab is a chimeric monoclonal antibody that targets the CD20 molecule on B-cells.[3] It is a US Food and Drug Administration approved drug for lymphoma, rheumatoid arthritis, chronic lymphocytic leukemia and Wegener's granulomatosis but has been also used off-label in severe and refractory pemphigus since 2002.[4]
The primary objective of this retrospective study was to assess the efficacy and adverse effects of rituximab in the treatment of pemphigus patients, most of whom were refractory to conventional therapy. The secondary objective was to measure the cumulative dose of corticosteroids required in these patients.
Methods
We reviewed the records of 25 patients with pemphigus who were treated with rituximab infusion in our department from March 2012 to January 2014. All patients were from the central, Northern and Eastern parts of India. Twentyone patients were recalcitrant to other immunosuppressive agents but in four patients, rituximab was used as first line therapy.
Skin biopsy, Tzanck smear, direct immunofluorescence and serum anti-desmoglein autoantibody levels [MESACUP desmoglein test Dsg1 and Dsg3 ELISA kits (RG-7880EC-D and RG-7880EC-D), MBL international corporation,≥20U/ml=positive] were done at baseline. A Mantoux test, viral markers for hepatitis B surface antigen, anti-hepatitis C virus, enzyme-linked immunosorbent assay for human immunodeficiency virus and routine biochemical investigations were also performed. After premedication (100 mg of hydrocortisone intravenously, 22.75 mg of pheniramine maleate intravenously and 500 mg of paracetamol orally), rituximab infusion was given under strict monitoring over 5–6 hours. The rate of infusion was initially 50 ml/hour, escalated by 50 ml/hour every 30 min upto a maximum infusion rate of 400 ml/hour. Twenty one patients received two doses of 1 gm of rituximab, 2 weeks apart. One patient received three doses of a 500 mg infusion at intervals of 2 weeks, four such doses were given to two other patients and one patient received four doses of 640 mg at intervals of 2 weeks. Oral prednisolone was administered at a dosage up to 0.5 mg/kg of body weight and tapered over the next 3–4 months according to the disease activity. However, other immunosuppressive agents such as cyclophosphamide and azathioprine, were continued for one more year after clinical remission and the stoppage of oral prednisolone.
Observation periods and end points
The early endpoint was time to disease control which was defined as the time at which new lesions ceased to form and established lesions began to heal.
Among the late endpoints, complete remission was defined as the absence of new or established lesions for at least 2 months. Partial remission was defined by the presence of transient new lesions that healed within 1 week with or without minimal therapy, including topical steroids. Relapse was characterized by the appearance of 3 or more new lesions in a month that did not heal spontaneously within 1 week or by the extension of established lesions, in a patient who had achieved disease control.[5]
Results
There were 25 patients with pemphigus comprising 17 men and 8 women with a mean age of 44.47 years (23–72 years); the duration of disease ranged from 3 months to 13 years (mean duration: 41.99 months). Only cutaneous involvement was present in four patients while in 21 patients, both the skin and oral mucosa were involved. The mean body surface area involved was 7.05%. Based on the histological, immunofluorescence findings and anti-desmoglein autoantibody levels (n = 15), a diagnosis of pemphigus vulgaris was made in 21 patients, and pemphigus foliaceus in four [Table - 1].
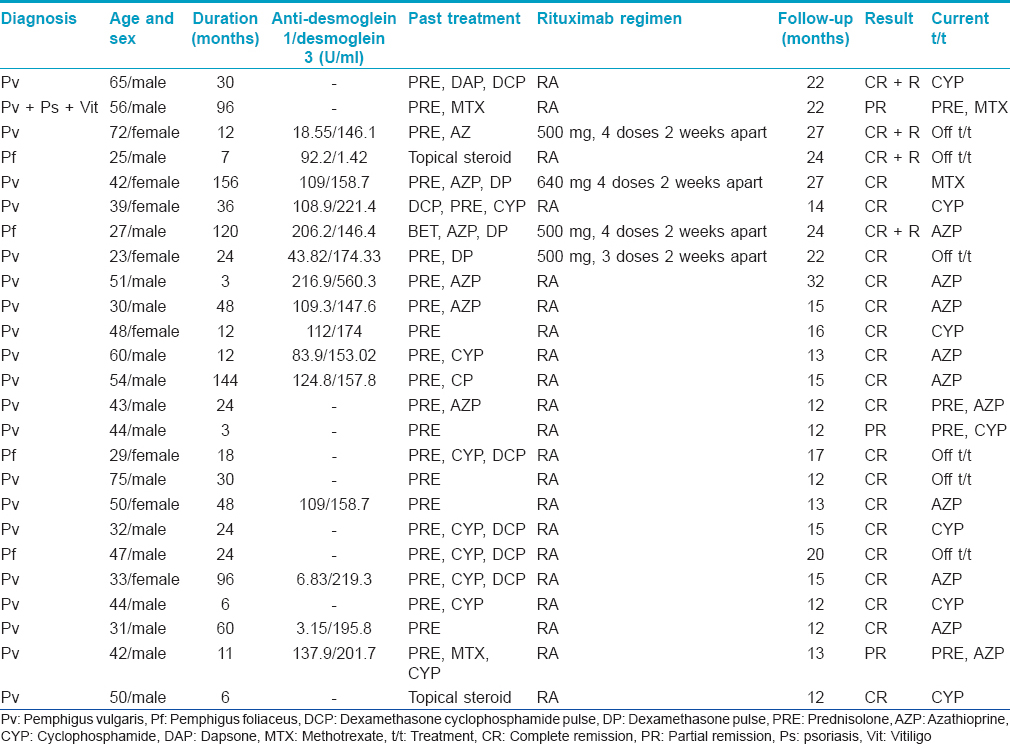
The follow-up period after the last rituximab infusion ranged from 12 to 32 months (mean: 17.51 months). Complete remission was seen in 22 (88%) out of 25 patients [Figure - 1] and [Figure - 2]. A partial response was noted in 3 (12%) patients including one patient who had associated chronic plaque psoriasis and vitiligo vulgaris. The mean time to disease control was 1.10 months and time to complete remission was 4.36 months. The mean duration of follow-up for the 21 recalcitrant cases was 48.6 months (range: 12–132). Fifteen patients had already received several cycles of monthly dexamethasone or dexamethasone cyclophosphamide pulse therapy (range: 12–72, mean: 19.8 cycles) while another five patients had received high doses of oral steroids and other immunosuppressive agents such as azathioprine, cyclophosphamide and methotrexate for periods greater than a year. Ten out of 15 patients who had received intravenous pulsed therapy had complete remission with this initial treatment but all of them relapsed after a mean duration of 9.5 months (range: 2–24 months); the other five patients could only achieve a partial remission.
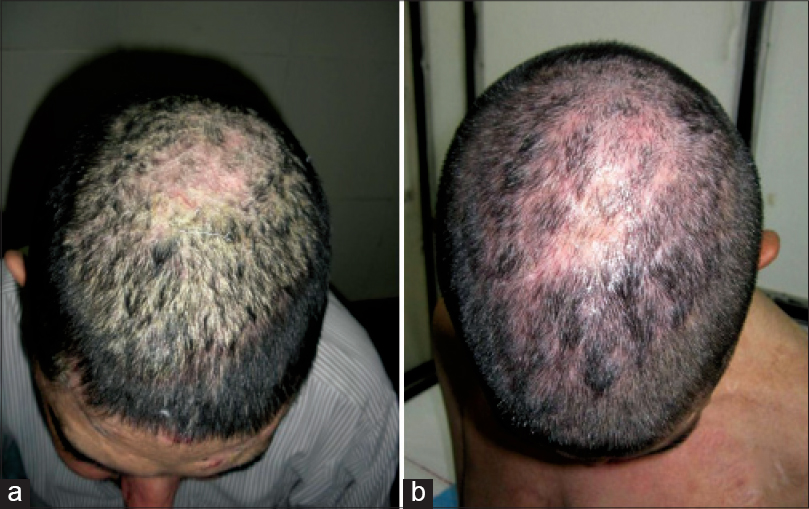 |
| Figure 1: (a and b) Response of recalcitrant scalp lesion to rituximab |
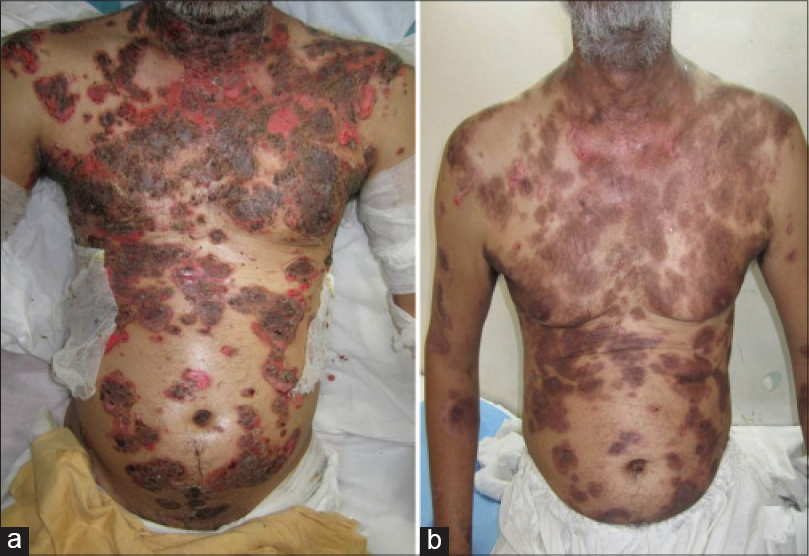 |
| Figure 2: (a and b) Response of extensive pemphigus within 2 weeks of rituximab infusion |
Four (16%) patients experienced relapse after a mean duration of 11.75 months from the last rituximab infusion. Three of these relapses were in previously recalcitrant cases and the relapse occurred within 1 month of the discontinuation of oral prednisolone. One new patient was not on any other medication prior to relapse. Relapses were mild in all the patients and resolved within 1 month without the need for additional medication, except for 50 mg of azathioprine twice daily in one patient who was not on any immunosuppressive agent. Of 23 patients (19 recalcitrant, 4 new), who were on oral prednisolone at the time of rituximab infusion, discontinuation of prednisolone was possible in 19 patients (16 recalcitrant, 3 new), with tapering of doses in the four patients. The mean total dose of oral prednisolone after rituximab infusion was 3535.64 mg (range 465–12,000 mg).
No immediate complications were seen. Exacerbation of the disease was noted in two patients after the first dose of rituximab and no specific predisposing or precipitating factor could be found in either of these two patients. The antibody titers could not be repeated during this period. However, the exacerbation was mild and required only an increase in the dose of oral steroids in one patient and an additional dexamethasone pulse therapy cycle in the other. These patients responded well to the second dose of rituximab. Pneumonia leading to acute respiratory distress syndrome and cellulitis developed in one patient each, but resolved with oral and intravenous antibiotics over the following 2 weeks.
Discussion
Rituximab has been found effective in the treatment of recalcitrant pemphigus vulgaris. The two most common dosing protocols described for rituximab are the lymphoma and rheumatoid arthritis protocols. The lymphoma protocol comprises four weekly infusions of 375 mg/m2 whereas two infusions of 1000 mg, 2 weeks apart are given in the rheumatoid arthritis protocol.[6],[7],[8],[9],[10],[11] A mixed protocol has also been used in some studies [Table - 2].
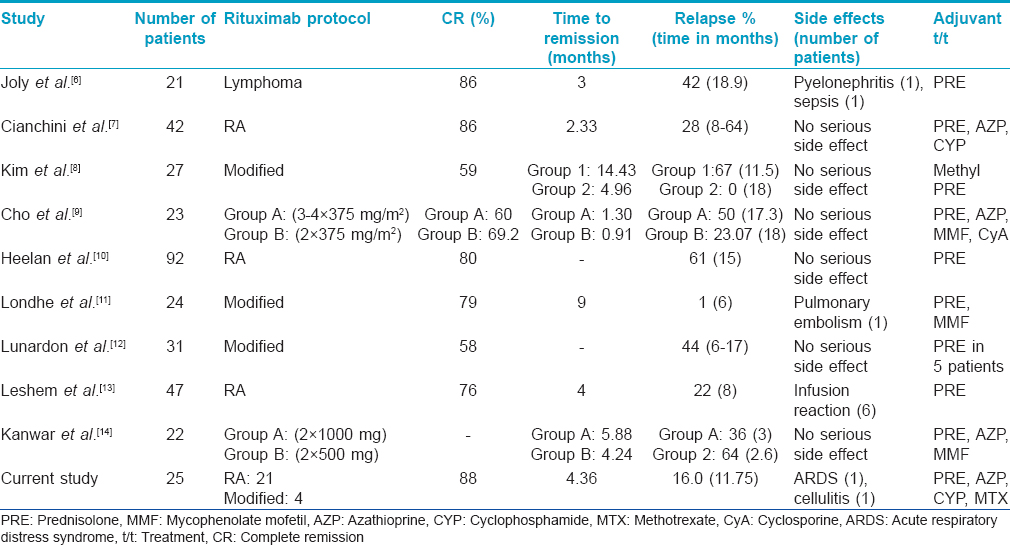
Lunardon et al. treated 31 pemphigus patients using rituximab (rheumatoid arthritis protocol: 16, lymphoma protocol: 15); of these, 18 (58%) patients achieved complete remission.[12] Leshem et al. used the rheumatoid arthritis protocol of rituximab infusion in 47 pemphigus patients and noted complete remission in 76% of the cases.[13] Relapse occurred in 22% of the patients at a median time of 8 months but 75% of the relapsing patients achieved remission with additional cycles. A comparatively higher number of patients in our study showed complete (88%) and partial remission (12%) whereas the relapse rate was lower (16%). No statistically significant association could be established between the remission rate and age of the patient, the body surface area involved or the baseline anti-desmoglein autoantibody levels. Pemphigus foliaceus patients were found to have an increased chance of relapse when compared to pemphigus vulgaris, although the number of cases was too small to draw a conclusion. In a review of 42 publications on 272 patients of pemphigus treated with rituximab, Zakka et al. compared the efficacy and adverse effects of different protocols. The total number of patients treated by the lymphoma protocol was 180 while 92 patients were treated with the rheumatoid arthritis protocol and its modification.[15] The mean follow-up for patients in the lymphoma protocol was 15.44 months and for the rheumatoid arthritis protocol was 21.04 months. The authors concluded that the lymphoma protocol produces a lower response rate (66.6% vs. 75%), lower rate of relapse (22.8% vs. 35.8%) and lower rate of serious infections (3.9% vs. 15.2%) but a higher mortality rate (2.2% vs. 1.1%) compared to the rheumatoid arthritis protocol. A recent study of 155 pemphigus patients concluded that the standard lymphoma and rheumatoid arthritis regimens are almost equally effective.[16] Due to the small number of patients treated with the lymphoma protocol in our study, no definitive conclusion regarding comparative efficacy could be drawn.
We used rituximab in an attempt to reduce the cumulative dose of oral steroids in pemphigus patients. In our patients, the mean total dose of oral prednisolone with rituximab infusion was 3535.64 mg (range 465–12,000 mg) which is much less than that reported with other adjuvants in pemphigus patients.[17],[18] A randomized controlled open-label trial of four treatment regimens for 120 pemphigus vulgaris patients reported a mean total dose of prednisolone of 11,631 mg, 7712 mg, 9798 mg and 8276 mg in the following 4 groups: prednisolone alone, prednisolone with azathioprine, prednisolone with mycophenolate mofetil and prednisolone with intravenous cyclophosphamide pulse therapy, respectively.[17]In a study conducted in our department, 60 pemphigus patients were divided into two groups to receive either daily oral prednisolone or intravenous cyclophosphamide pulse therapy and prednisolone for 1 year. The cumulative dose of oral prednisolone was 7450–17320 mg (mean = 10540 mg) in group 1 and 5120–19160 mg (mean = 8990 mg) in group 2.[18] In a randomized trial by Kanwar et al., 22 patients with pemphigus were randomized to receive either 2 doses of 1000 mg rituximab or 500 mg rituximab at 0 and 2 weeks. The total cumulative dose of prednisolone was 2432.73 mg in group A and 3006.50 mg in group B.[14] Thus, rituximab resulted in control of pemphigus at a lower initial dose of oral prednisolone and hence the total cumulative dose was reduced.
Concerns about rituximab safety stem from multiple studies because serious fatal side effects such as sepsis, pyelonephritis, infusion reactions and other systemic infections have been noted.[8],[13] Mild self-limiting side effects have also been noted in a few other studies which include influenza-like symptoms, gastrointestinal upset, herpes zoster and tachycardia with chest pain.[8] In our study, 10.5% of the patients developed serious infections after rituximab infusion which is lower than the 15.2% reported in a review of the rheumatoid arthritis protocol.[15] We noticed a paradoxical exacerbation of disease activity after the first rituximab infusion in two patients, a feature that has also been observed previously.[13] No specific mechanism can be postulated for this phenomenon but the initial destruction of protective antibodies by rituximab in some genetically predisposed individuals can be considered as a possible explanation. These two patients had good improvement in disease activity after their subsequent rituximab infusions. The major limitations of our study were the small sample size, retrospective nature of the study, lack of a validated severity score, unavailability of follow-up anti-desmoglein auto-antibodies levels and B cell markers.
Conclusion
Our experience shows that rituximab is an effective form of therapy for pemphigus. We found it useful in long-standing as well as early disease. Severe side effects are rare but careful monitoring should be done in all patients to avoid complications. Further larger studies and randomized controlled trials would be valuable to determine the safety and efficacy of this drug as a first-line agent in pemphigus.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
| 1. |
Kanwar AJ, De D. Pemphigus in India. Indian J Dermatol Venereol Leprol 2011;77:439-49.
[Google Scholar]
|
| 2. |
Pasricha JS, Khaitan BK, Raman RS, Chandra M. Dexamethasone-cyclophosphamide pulse therapy for pemphigus. Int J Dermatol 1995;34:875-82.
[Google Scholar]
|
| 3. |
Salopek TG, Logsetty S, Tredget EE. Anti-CD20 chimeric monoclonal antibody (rituximab) for the treatment of recalcitrant, life-threatening pemphigus vulgaris with implications in the pathogenesis of the disorder. J Am Acad Dermatol 2002;47:785-8.
[Google Scholar]
|
| 4. |
Heizmann M, Itin P, Wernli M, Borradori L, Bargetzi MJ. Successful treatment of paraneoplastic pemphigus in follicular NHL with rituximab: Report of a case and review of treatment for paraneoplastic pemphigus in NHL and CLL. Am J Hematol 2001;66:142-4.
[Google Scholar]
|
| 5. |
Murrell DF, Dick S, Ahmed AR, Amagai M, Barnadas MA, Borradori L, et al. Consensus statement on definitions of disease, end points, and therapeutic response for pemphigus. J Am Acad Dermatol 2008;58:1043-6.
[Google Scholar]
|
| 6. |
Joly P, Mouquet H, Roujeau JC, D'Incan M, Gilbert D, Jacquot S, et al. A single cycle of rituximab for the treatment of severe pemphigus. N Engl J Med 2007;357:545-52.
[Google Scholar]
|
| 7. |
Cianchini G, Lupi F, Masini C, Corona R, Puddu P, De PitÜ O. Therapy with rituximab for autoimmune pemphigus: Results from a single-center observational study on 42 cases with long-term follow-up. J Am Acad Dermatol 2012;67:617-22.
[Google Scholar]
|
| 8. |
Kim JH, Kim YH, Kim MR, Kim SC. Clinical efficacy of different doses of rituximab in the treatment of pemphigus: A retrospective study of 27 patients. Br J Dermatol 2011;165:646-51.
[Google Scholar]
|
| 9. |
Cho HH, Jin SP, Chung JH. Clinical experiences of different dosing schedules of rituximab in pemphigus with various disease severities. J Eur Acad Dermatol Venereol 2014;28:186-91.
[Google Scholar]
|
| 10. |
Heelan K, Al-Mohammedi F, Smith MJ, Knowles S, Lansang P, Walsh S, et al. Durable remission of pemphigus with a fixed-dose rituximab protocol. JAMA Dermatol 2014;150:703-8.
[Google Scholar]
|
| 11. |
Londhe PJ, Kalyanpad Y, Khopkar US. Intermediate doses of rituximab used as adjuvant therapy in refractory pemphigus. Indian J Dermatol Venereol Leprol 2014;80:300-5.
[Google Scholar]
|
| 12. |
Lunardon L, Tsai KJ, Propert KJ, Fett N, Stanley JR, Werth VP, et al. Adjuvant rituximab therapy of pemphigus: A single-center experience with 31 patients. Arch Dermatol 2012;148:1031-6.
[Google Scholar]
|
| 13. |
Leshem YA, Hodak E, David M, Anhalt GJ, Mimouni D. Successful treatment of pemphigus with biweekly 1-g infusions of rituximab: A retrospective study of 47 patients. J Am Acad Dermatol 2013;68:404-11.
[Google Scholar]
|
| 14. |
Kanwar AJ, Vinay K, Sawatkar GU, Dogra S, Minz RW, Shear NH, et al. Clinical and immunological outcomes of high- and low-dose rituximab treatments in patients with pemphigus: A randomized, comparative, observer-blinded study. Br J Dermatol 2014;170:1341-9.
[Google Scholar]
|
| 15. |
Zakka LR, Shetty SS, Ahmed AR. Rituximab in the treatment of pemphigus vulgaris. Dermatol Ther (Heidelb) 2012;2:17.
[Google Scholar]
|
| 16. |
Amber KT, Hertl M. An assessment of treatment history and its association with clinical outcomes and relapse in 155 pemphigus patients with response to a single cycle of rituximab. J Eur Acad Dermatol Venereol 2015;29:777-82.
[Google Scholar]
|
| 17. |
Chams-Davatchi C, Esmaili N, Daneshpazhooh M, Valikhani M, Balighi K, Hallaji Z, et al. Randomized controlled open-label trial of four treatment regimens for pemphigus vulgaris. J Am Acad Dermatol 2007;57:622-8.
[Google Scholar]
|
| 18. |
Sharma VK, Khandpur S. Evaluation of cyclophosphamide pulse therapy as an adjuvant to oral corticosteroid in the management of pemphigus vulgaris. Clin Exp Dermatol 2013;38:659-64.
[Google Scholar]
|
Fulltext Views
6,812
PDF downloads
3,759





