Translate this page into:
Comparative efficacy and safety of JAK/TYK2 inhibitors and other oral drugs for moderate-to-severe plaque psoriasis: Systematic review and network meta-analysis
Corresponding author: Huichun Su, Department of Dermatology and Venerology, Fujian Medical University Union Hospital, Fuzhou, China. suhuichun@163.com
-
Received: ,
Accepted: ,
How to cite this article: Zheng Y, Han Y, Chen J, Huang J, Zhu C, Lin L, et al. Comparative efficacy and safety of JAK/TYK2 inhibitors and other oral drugs for moderate-to-severe plaque psoriasis: Systematic review and network meta-analysis. Indian J Dermatol Venereol Leprol. 2024;90:590-8. doi: 10.25259/IJDVL_775_2023
Abstract
Background
Janus kinase (JAK)/tyrosine kinase 2 (TYK2) inhibitors are novel treatments for moderate-to-severe plaque psoriasis.
Objective
To perform a network meta-analysis to compare the efficacy and safety of TYK2 inhibitors with other oral drugs in moderate-to-severe psoriasis.
Methods
Eligible randomised clinical trials (RCTs) were identified from public databases (published before November 2, 2023). Random-effect frequentist network meta-analysis was performed with ranking based on the surface under the cumulative ranking curve (SUCRA) of Physician’s Global Assessment of “clear” or “almost clear” (PGA 0/1), 75% reduction from baseline in Psoriasis Area and Severity Index (PASI-75).
Results
Twenty RCTs containing 7,564 patients with moderate-to-severe psoriasis were included. Deucravacitinib at all dose levels (except for 3 mg every other day) and tofacitinib (10 mg BID) ranked best in achieving PGA 0/1 and PASI-75 at 12– 16 weeks. Tofacitinib (10 mg BID) was considered the most unsafe. Analysis of Ranking according to efficacy and safety showed deucravacitinib (3 mg QD and 3 mg BID) was the best treatment.
Analysis of Ranking according to efficacy and safety showed deucravacitinib (3 mg QD and 3 mg BID) was the best treatment.
Limitation
Insufficiency of eligible data and no long-term follow-up data.
Conclusion
Deucravacitinib showed superior efficacy and safety for treating moderate-to-severe psoriasis over other included drugs.
Keywords
psoriasis
biologics
deucravacitinib
JAK inhibitors
TYK2
tofacitinib.
Introduction
Psoriasis is a chronic, immune-mediated disorder with cutaneous and systemic manifestations and substantial negative effects on a patient’s quality of life.1 Plaque psoriasis (PSO), also known as psoriasis vulgaris, is the most common form of psoriasis.2 PSO is characterised by sharply demarcated plaque, and erythematous or scaly patches that usually occur on the surface of the extensor muscles but can also affect the intertriginous areas, palms, soles of the feet, and nails.3 It has been reported that an increased incidence of psoriatic arthritis and cardiometabolic, hepatic, and psychological comorbidities in patients affected by moderate-to-severe PSO require a holistic and multidisciplinary approach to care.4
Moderate-to-severe PSO often requires systemic treatment. It is worth noting that oral medications improve patient compliance because of their convenience. Apremilast, a phosphodiesterase 4 (PDE4) inhibitor, was the first oral drug approved for treating PSO by the US Food and Drug Administration (FDA) since 1996.5 Inhibition of PDE4 by densifying intracellular cyclic adenosine monophosphate affects intracellular signal transduction.6 Another approved oral drug, fumarate, exerts its anti-psoriatic effect by regulating antioxidant defence and inflammatory pathways in psoriasis.7 Other oral drugs included Janus kinase (JAK) inhibitors (JAKi) and immunity inhibitors such as methotrexate and cyclosporine. Despite some evidence supporting the efficacy of the abovementioned drugs, the relative non-specificity and side effects hinder their application in PSO therapy.8 The current research focuses on tyrosine kinase 2 (TYK2) which belongs to the JAK family along with three other subtypes: JAK1/2/3.9 The inhibition of TYK2 signalling breaks the link between interleukin (IL)-23 and IL-17 production which inhibits critical PSO signalling.10–12 Deucravacitinib (DEU) which is a first-in-class, highly selective inhibitor, is the only approved oral drug among JAK/TYK2 inhibitors.13 Two other TYK2 inhibitors (TYK2i), ropsacitinib and brepocitinib, are currently in clinical development.14–15
To date, comparisons of JAK/TYK2 inhibitors have not been updated. Network meta analysis adds further weight to evidence of the best treatment through a combination of direct and indirect comparison.16 Therefore, we conducted a systematic review and Network meta-analysis to compare the efficacy and safety of JAK/TYK2 inhibitors with other oral drugs for patients with moderate-to-severe PSO and provided a reliable basis for clinical application.
Methods
The systematic review and network meta-analysis in this study were conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-analyses Extension Statement for Network Meta-Analyses (PRISMA-NMA) guidelines.17 No ethical approval or informed consent was required because this network meta-analysis was conducted using published studies. This network meta-analysis was already registered in the PROSPERO database (CRD42023404337).
Search strategies
We systematically searched the PubMed, EMBASE, Web of Science, MEDLINE, Clinical Trials.gov (CT.gov), and Cochrane Central Register of Controlled Trials (CENTRAL) databases from database initiation to November 2, 2023, with no restrictions of date or language. The search terms were related to PSO, randomised controlled trials (RCTs), and interventions. The search strategy is provided in the supplementary material. To prevent the omission of relevant studies, references for the included RCTs were also inspected in the process of the systematic search.
Eligibility criteria and study selection
Included trials needed to meet the following criteria: (1) individuals who had been diagnosed with moderate-to-severe PSO for at least 6 months and were treated with oral drugs for 12 weeks or longer; (2) phase II or III RCTs; (3) studies that provided any outcome of interesting, such as Physician’s Global Assessment (PGA), baseline psoriasis area and severity index (PASI) and treatment-emergent adverse events. We excluded studies with insufficient information and combination therapies. Any case series, case reports, cohorts (retrospective or prospective), review articles, meta-analyses, letters, and brief reports were also omitted.
Two reviewers (Y.Z. and J.C.) screened the studies independently by title, abstract, and full text, according to the inclusion and exclusion criteria. In the event of a disagreement, the third reviewer (H.S.) helped reach a consensus.
Data extraction and outcome measures
For our network meta-analysis, we extracted the following predefined variables: authors, year of publication, study type, name and phase of the study, clinical trial number, baseline patient characteristics (age, gender, weight, baseline PASI), treatment regimens, follow-up duration and outcomes of interest. The outcomes included the PGA of ‘clear’ or ‘almost clear’ (PGA 0/1), 75% reduction from baseline in the PASI (PASI-75), treatment-emergent adverse events, and discontinuation owing to adverse events at 12–16 weeks.
Statistical analysis
All analysis of data was processed by using STATA software (version 17) with the metan, mvmeta, and network packages. Traditional pairwise meta-analysis (direct meta-analysis) was used to compare multiple interventions and placebo. Network meta-analysis based on a frequentist framework was implemented to assess the efficacy and safety of all treatments by providing an additional indirect comparison. Statistical heterogeneity was tested using the Cochrane Q test and quantified by the I2 statistic. The random effects model was adopted considering the heterogeneity of different trials (I2 > 50%). The network map was constructed to show the connection among the studies. Comparison-adjusted funnel plots and Egger’s test were used to assess potential publication bias.18 The surface under the cumulative ranking (SUCRA) curve, ranging from 0% to 100%, was calculated with a higher SUCRA value indicating that the drugs are deemed to be the best treatment with more certainty. In addition, cumulative probability plots were generated to visualise treatment hierarchies.19 The efficacy and safety outcomes in this network meta-analysis were dichotomous variables and the effect sizes were present by calculating the relative risk (RR) and its 95% confidence interval (CI) as well as the number needed to treat which is obtained by taking the reciprocal of the response difference of different drugs versus placebo. We also examine the robustness of the model by sensitivity analyses based on the ‘leave-one-out’ methods. Consistency was tested by using the global inconsistency test and node-splitting method. P < 0.05 indicates the statistical significance of the analysis.
Assessment of risk of bias
Two independent reviewers (Y.Z. and J.C.) conducted a quality assessment according to the guidelines of the Cochrane Handbook for Systematic Reviews of Interventions to evaluate the risk of bias of included trials based on the following items: randomisation of sequence generation; allocation concealment; blinding for patients, personnel and outcome measures; missing outcome data; selective reporting; and other sources of bias.20 In addition a third reviewer (H.S.) helped to resolve any disagreements.
Results
Search results and trial characteristics
Twenty RCTs containing 7,564 moderate-to-severe PSO patients were included in our network meta-analysis.6,21–38 Ten oral drugs including six TYK2/JAK inhibitors (Deucravacitinib, brepocitinib, ropsacitinib, tofacitinib, solcitinib, baricitinib, methotrexate, apremilast, cyclosporine, and fumarate was reported for 4, 1, 1, 6, 1, 1, 3, 3, 1 and 1 studies, respectively) were finally included. The PRISMA flowchart and detailed search strategy are shown in the Supplement file. The study characteristics are summarised in eTable 1. The network plots of different comparisons for all outcomes are shown in Figure 1a–1d. The risk of bias for 16 RCTs was assessed to be low, and one study was reckoned to be at high risk of bias [eFigure 1 and eFigure 2 in the Supplementary files]. No detectable inconsistency was found [eTable 2].
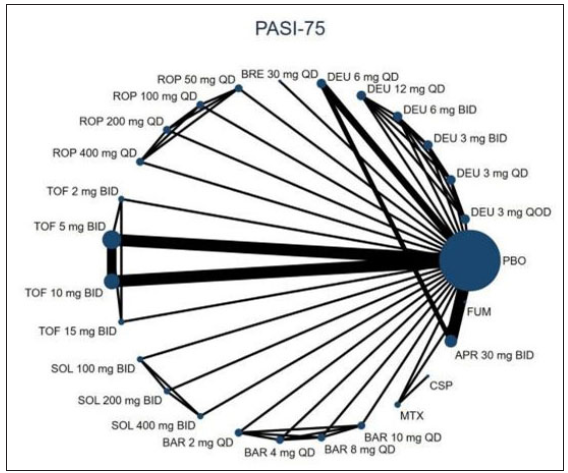
- The evidence network plot of all papers about different treatments. PASI-75. (DEU: deucravacitinib; BRE: brepocitinib; ROP: ropsacitinib; TOF: tofacitinib; SOL: solcitinib; BAR: baricitinib; MTX: methotrexate; APR: apremilast; CSP: cyclosporine; FUM: fumarate.)
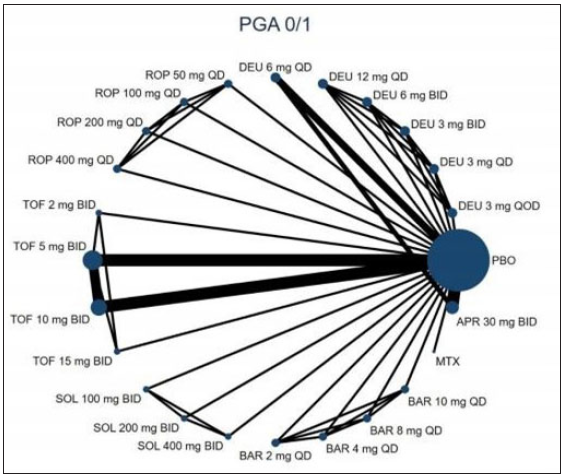
- The evidence network plot of all papers about different treatments. PGA 0/1. (DEU: deucravacitinib; BRE: brepocitinib; ROP: ropsacitinib; TOF: tofacitinib; SOL: solcitinib; BAR: baricitinib; MTX: methotrexate; APR: apremilast.)
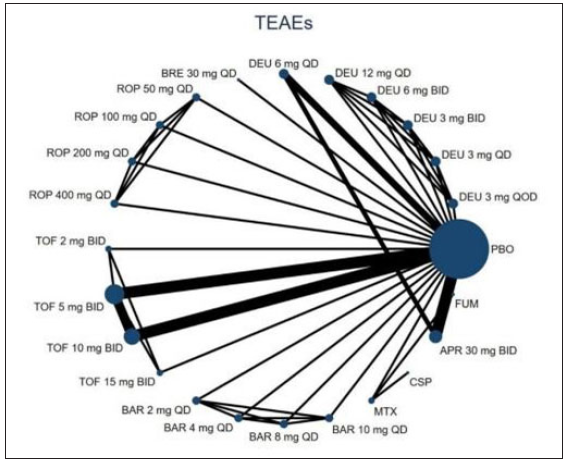
- The evidence network plot of all papers about different treatments. (TEAEs: Treatment-emergent adverse events; DEU: deucravacitinib; BRE: brepocitinib; ROP: ropsacitinib; TOF: tofacitinib; SOL: solcitinib; BAR: baricitinib; MTX: methotrexate; APR: apremilast; CSP: cyclosporine; FUM: fumarate.)
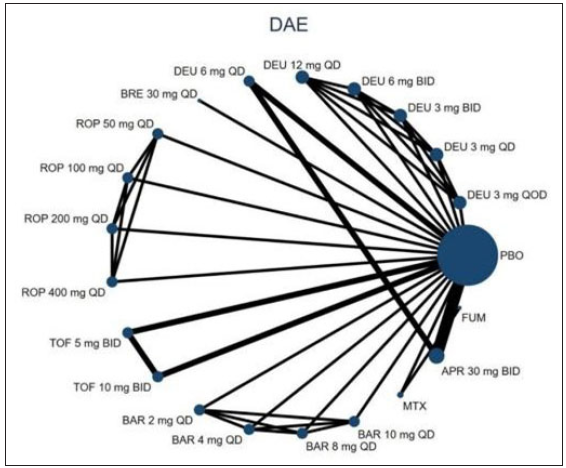
- The evidence network plot of all papers about different treatments. Line thicknesses corresponded to the number of trials and node sizes indicated the total sample sizes for treatments. (DAE: discontinuation owing to adverse events; DEU: deucravacitinib; BRE: brepocitinib; ROP: ropsacitinib; TOF: tofacitinib; SOL: solcitinib; BAR: baricitinib; MTX: methotrexate; APR: apremilast; FUM: fumarate.)
Efficacy outcomes
PGA 0/1
Seventeen RCTs reported the PGA 0/1 data at 12–16 weeks. Compared to the placebo, all included treatments except for solcitinib and baricitinib resulted in a higher proportion of patients with PGA 0/1 at both 12 and 16 weeks (p < 0.01) [Figure 2]. Notably, the highest RR to achieve PASI-75 was deucravacitinib 3 mg twice a day (BID) (RR = 11.33, 95% CI = 3.75–34.24, the number needed to treat = 1.45). Overall, the SUCRA values revealed that deucravacitinib (3 mg BID, 12 mg once daily (QD), and 6 mg BID) had the best three PGA 0/1 response rates at 12–16 weeks (SUCRA = 0.889, 0.887, and 0.828) [Figure 3a]. Based on the network meta-analysis, deucravacitinib (3 mg BID, 6 mg BID, 6 mg QD, and 12 mg QD) and tofacitinib (10 mg BID and 15 mg BID) were the best treatments associated with improved PGA 0/1 at weeks 12–16 over most of the other oral drugs [eTable 3].
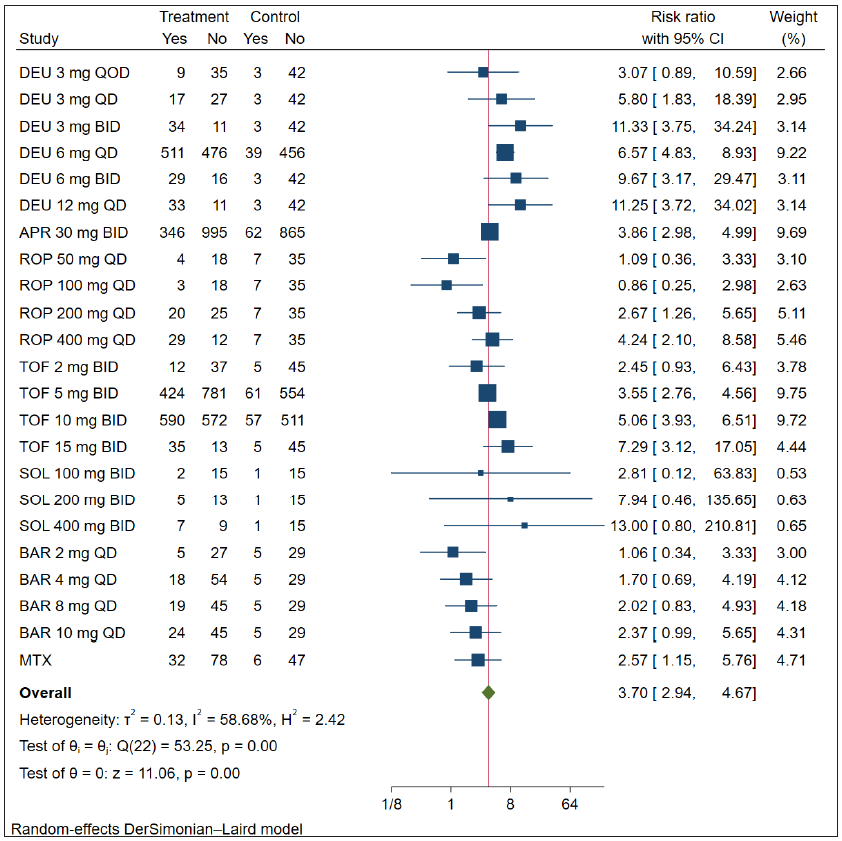
- Relative risk of achieving PGA 0/1 response of oral drugs in the treatment of plaque psoriasis versus placebo. (DEU: deucravacitinib; ROP: ropsacitinib; TOF: tofacitinib; SOL: solcitinib; BAR: baricitinib; MTX: methotrexate; APR: apremilast; CI: Confidence interval; PGA: Physician’s Global Assessment)
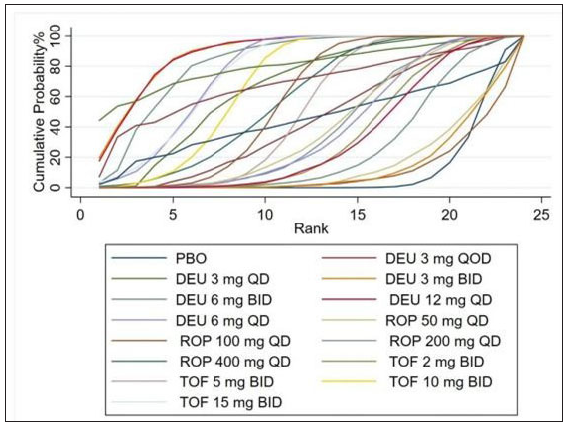
- Rank of the cumulative probabilities for PGA 0/1. (DEU: deucravacitinib; ROP: ropsacitinib; TOF: tofacitinib; PBO: placebo.)
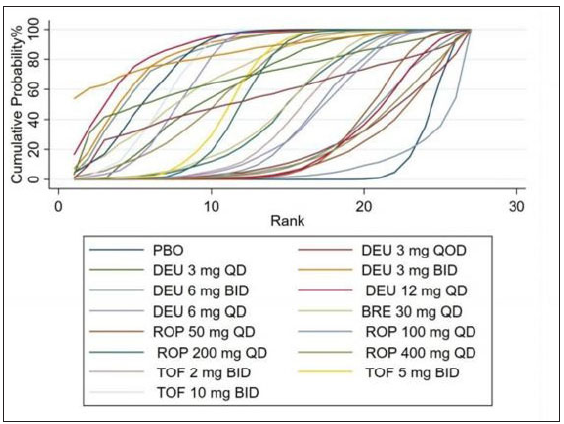
- Rank of the cumulative probabilities for PASI-75. (DAE: discontinuation owing to adverse events; DEU: deucravacitinib; BRE: brepocitinib; ROP: ropsacitinib; TOF: tofacitinib; PBO: placebo.)
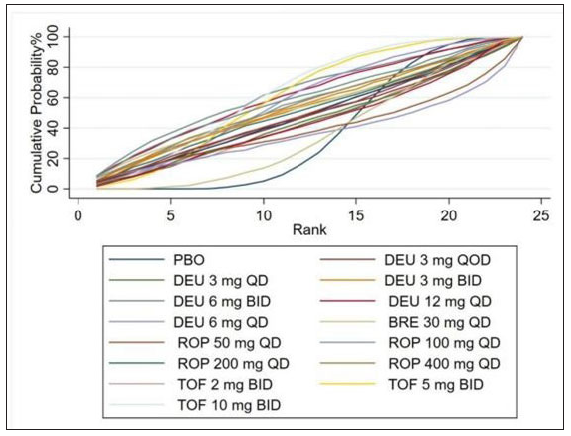
- Rank of the cumulative probabilities for treatment-emergent adverse events. (DEU: deucravacitinib; BRE: brepocitinib; ROP: ropsacitinib; TOF: tofacitinib; PBO: placebo.)
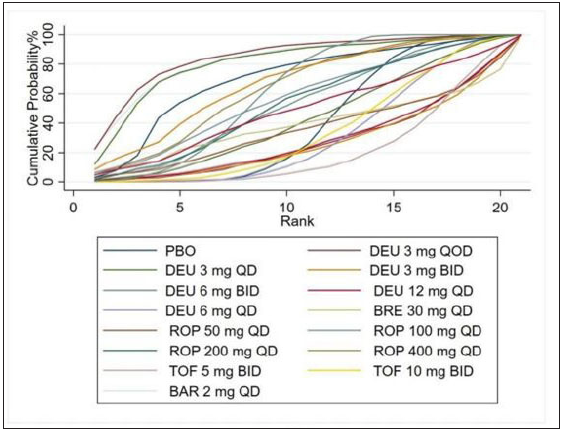
- Rank of the cumulative probabilities for DAE. (DAE: discontinuation owing to adverse events; DEU: deucravacitinib; BRE: brepocitinib; ROP: ropsacitinib; TOF: tofacitinib; BAR: baricitinib; PBO: placebo.)
PASI-75
All 20 RCTs reported PASI-75 data at 12–16 weeks. All JAK/TYK2 inhibitors resulted in significantly higher PASI-75 compared with the placebo (P < 0.01) (eFigure 3). And, tofacitinib 15 mg BID had the highest efficacy (RR = 32.65, 95% CI = 4.64–229.73, the number needed to treat = 1.59). Overall, the SUCRA values revealed that deucravacitinib (3 mg BID and 12 mg QD) and solcitinib (400 mg BID) were most likely to be the best treatments in terms of PASI-75 (SUCRA = 0.838, 0.875, and 0.850) [Figure 3b]. In addition, the network meta-analysis also demonstrated that deucravacitinib (3 mg BID, 6 mg QD, 6 mg BID, and 12 mg QD) and tofacitinib (5 mg BID, 10 mg BID, and 15 mg BID) were associated with improved PASI-75 at weeks 12–16 compared with other oral drugs [eTable 4].
Safety
Treatment-emergent adverse events
Nineteen included RCTs reported treatment-emergent adverse events. As illustrated in Figure 4, the treatment-emergent adverse events for all treatments were non-significantly different from those of the placebo except deucravacitinib (6 mg BID and 12 mg QD) (RR = 1.57, 95% CI = 1.14–2.16, the number needed to treat = 3.45; RR = 1.51, 95% CI = 1.09–2.10, the number needed to treat = 3.85), tofacitinib (5 mg BID) (RR = 1.59, 95% CI = 1.40–1.81, the number needed to treat = 4.55) and tofacitinib (10 mg BID) (RR = 1.85, 95% CI = 1.60–2.13, the number needed to treat = 3.45). SUCRA analysis showed that tofacitinib at 10 mg BID had the best possibility to cause treatment-emergent adverse events (SUCRA = 0.634), followed by deucravacitinib at 6 mg BID (SUCRA = 0.633) [Figure 3c]. According to network meta-analysis, all the treatments showed similar probabilities of Treatment-emergent adverse events with negative RRs [eTable 5].
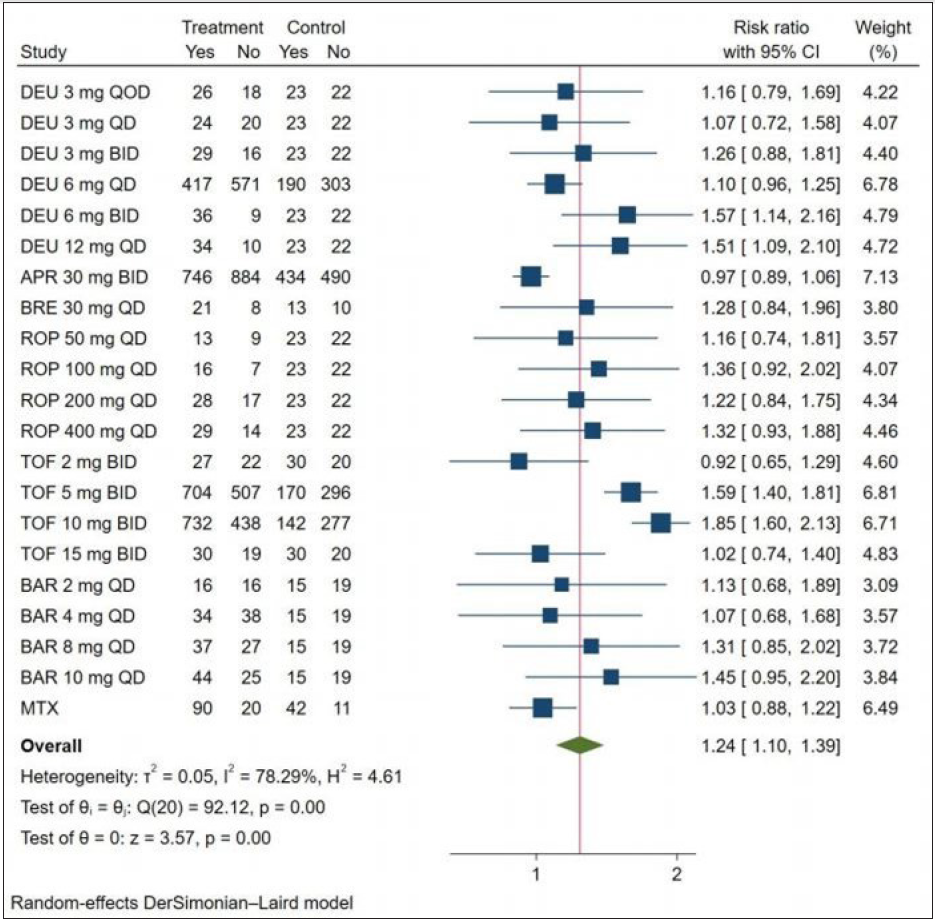
- Relative risk of achieving treatment-emergent adverse events response of oral drugs in the treatment of plaque psoriasis versus placebo. (DEU: deucravacitinib; BRE: brepocitinib; ROP: ropsacitinib; TOF: tofacitinib; SOL: solcitinib; BAR: baricitinib; MTX: methotrexate; APR: apremilast.)
We also ranked the oral drugs according to their efficacy and safety. Deucravacitinib (3 mg QD and 3 mg BID) which appear in the upper right corner of [Figure 5] struck a good balance between efficacy and safety.
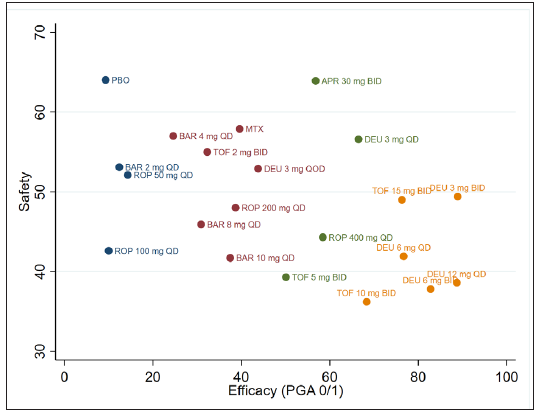
- Ranking of oral drugs according to safety and efficacy of PGA 0/1. (DEU: deucravacitinib; BRE: brepocitinib; ROP: ropsacitinib; TOF: tofacitinib; SOL: solcitinib; BAR: baricitinib; MTX: methotrexate; APR: apremilast; QD: once daily; BID: twice a day.)
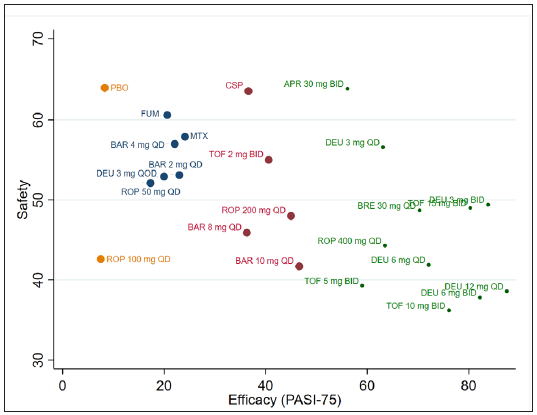
- Ranking of oral drugs according to safety and efficacy of PGA 0/1 at 12–16 weeks. (DEU: deucravacitinib; BRE: brepocitinib; ROP: ropsacitinib; TOF: tofacitinib; SOL: solcitinib; BAR: baricitinib; MTX: methotrexate; APR: apremilast; CSP: cyclosporine; FUM: fumarate; QD: once daily; BID: twice a day.)
Discontinuation owing to adverse events
Discontinuation owing to adverse events compared to placebo, treatment with the JAK/TYK2 inhibitors did not result in significantly different DAE risk but apremilast (30 mg BID) showed a positive RR (2.10, 95% CI = 1.36–3.25, the number needed to treat = 25) (eFigure 4). Based on the SUCRA values for DAE, baricitinib (8 mg QD) ranks best in safety risk (SUCRA = 0.847) [Figure 3d]. Moreover, our network meta-analysis supported that apremilast (30 mg BID) was the only treatment that had significantly lower safety than placebo [eTable 6].
Sensitivity analysis and publication bias assessment
Sensitivity analyses were performed to evaluate the robustness of the results by using ‘leave-one-out’ methods. As shown in eTable 7, regardless of which study was removed, the overall statistical significance remained consistent in both fixed- and random-effect models indicating the results were robust. On the other hand, we did not find publication bias in studies reporting PGA 0/1, PASI-75, treatment-emergent adverse events, and discontinuation owing to adverse events outcomes based on visually symmetric comparison-adjusted funnel plots and the Egger test results (P > 0.05) [eFigure 5].
Discussion
Through this network meta-analysis based on 20 trials with 7,564 moderate-to-severe PSO patients, we comparatively assessed three novel oral TYK2i and other oral agents. In terms of PASI-75 and PGA 0/1, deucravacitinib (except for 3 mg every other day and 3 mg QD) and tofacitinib (10 mg BID and 15 mg BID) exhibited significantly better efficacy than the other agents. Regarding safety, no significant differences in treatment-emergent adverse events were observed between the placebo and the JAK/TYK2 inhibitors except for deucravacitinib (6 mg BID and 12 mg QD), tofacitinib (5 mg BID and 10 mg BID). Comprehensive rankings based on efficacy and safety indicated that deucravacitinib (3 mg QD and 3 mg BID) struck a good balance.
Our results highlight the role of TYK2 in the pathogenesis of psoriasis and the therapeutic potential of TYK2i. A recent network meta-analysis compared TYK2i with apremilast and showed that TYK2i surpassed APR at certain doses which is consistent with our results.39 In addition, we also included immunity inhibitors — methotrexate and cyclosporine – showing their worse efficacy and a similar safety profile compared to TYK2i. The better efficacy of tofacitinib (a pan-JAK inhibitor) compared with solcitinib (a JAK1 inhibitor) and baricitinib (a JAK1 and JAK2 inhibitor) could be explained by its stronger ability to inhibit all JAKs rather than selectively targeting one or two JAKs. However, our data failed to support the superior clinical effects of ropsacitinib (a TYK2/JAK2 inhibitor) and BRE (a TYK2/JAK1 inhibitor) over deucravacitinib which may be the result of the weaker inhibition of TYK2 mediating the key IL-23/IL-17 axis.40
In the present study, the most unsafe treatment in terms of treatment-emergent adverse events was tofacitinib (10 mg BID) followed by deucravacitinib (6 mg BID) which is opposed to their better efficacy. Even so, all included JAK/TYK2 inhibitors did not lead to an increased risk of treatment discontinuation.
According to the long-term extension results of two large phases 3 52-week trials (POETYK PSO-1 and POETYK PSO-2), deucravacitinib has a low incidence of adverse events, the most common of which were nasopharyngitis, upper respiratory infections, headache, diarrhoea and nausea with opportunistic infections, systemic fungal or tuberculosis unreported.41–43 Notably, the safety profile is based on the results of using deucravacitinib at 6 mg QD which is the recommended daily dosage.44 Our network meta-analysis suggested that increasing the daily doses of deucravacitinib to 12 mg will result in an observational safety risk with the largest the number needed to treat of 3.85.
To date, tofacitinib was denied approval for psoriasis treatment in 2015 due to its side effects, although it was later approved for the treatment of psoriatic arthritis by the FDA. However, deucravacitinib received its first approval (in the USA on September 9, 2022) for the treatment of adults with moderate-to-severe PSO.45 We found that deucravacitinib treatment at 3 mg BID struck a good balance between short-term efficacy and safety. This might be because these TYK2i and especially deucravacitinib have high selectivity for TYK2, thereby reducing the negative safety outcomes associated with JAK1/2/3 inhibition (e.g., infection, hyperlipidaemia, and cytopenia) which suggests its core competency in oral drugs for PSO.46 Furthermore, the oral administration of deucravacitinib may increase patient compliance compared to other injectable biologics such as secukinumab by avoiding injection pain and the hassle of travelling to a clinic for injections.47 Despite its superiority, the cost of deucravacitinib will be a troublesome problem. According to the report, deucravacitinib is expected to cost up to $14,409 per patient per year in the absence of reimbursement.48 Considering the economic burden on patients with PSO, they may still give priority to traditional JAKi.
Although most RCTs included in this network meta-analysis were of high quality, we still encountered some limitations: (1) The follow-up periods ranging from 12 to 16 weeks are short. Thus, more well-designed multicentre RCTs with big sample sizes and long-term extension studies were needed to verify the safety profile of these drugs. (2) I think the RCT evidence on MTX is quite limited. Just like the authors say for newer TYK2 inhibitors. This could be the reason for apremilast being ranked better than MTX [see Figure 5]. As apremilast being superior to MTX does not match our clinical experience. (3) A certain degree of heterogeneity across studies was identified which may negatively affect the robustness and reliability of pooled results.
Overall, this network meta-analysis supported the efficacy of JAK/TYK2 inhibitors for moderate-to-severe PSO and the superior efficacy of TYK2i (deucravacitinib, BRE and ROP) especially deucravacitinib compared to JAKi (tofacitinib, solcitinib and baricitinib) and other oral drugs (methotrexate, apremilast, cyclosporine and FUM). More prospective studies should be conducted to assess the long-term efficacy and safety of TYK2i in psoriasis patients.
Declaration of patient consent
Patient’s consent not required as there are no patients in this study.
Financial support and sponsorship
The Investigator Initiation Fund Project of Fujian Medical University Union Hospital (2021XH026).
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
References
- Diagnosis and classification of psoriasis. Autoimmun Rev. 2014;13:490-5.
- [CrossRef] [PubMed] [Google Scholar]
- Pathophysiology, clinical presentation, and treatment of psoriasis: A review. JAMA. 2020;323:1945-60.
- [CrossRef] [PubMed] [Google Scholar]
- Psoriasis and comorbid diseases: Epidemiology. J Am Acad Dermatol. 2017;76:377-90.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Evolving utility of apremilast in dermatological disorders for off-label indications. Clin Exp Dermatol. 2022;47:2136-49.
- [CrossRef] [PubMed] [Google Scholar]
- Efficacy and safety of apremilast, an oral phosphodiesterase 4 inhibitor, in patients with moderate-to-severe plaque psoriasis over 52 weeks: A phase III, randomized controlled trial (ESTEEM 2) Br J Dermatol. 2015;173:1387-99.
- [CrossRef] [PubMed] [Google Scholar]
- Emerging therapeutic applications for fumarates. Trends Pharmacol Sci. 2021;42:239-54.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- A review on the safety of using JAK inhibitors in dermatology: clinical and laboratory monitoring. Dermatol Ther (Heidelb). 2023;13:729-49.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Selective Tyk2 inhibitors as potential therapeutic agents: a patent review (2015–2018) Expert Opin Ther Pat. 2019;29:137-49.
- [CrossRef] [PubMed] [Google Scholar]
- Tyrosine kinase 2 and Janus kinase‒signal transducer and activator of transcription signaling and inhibition in plaque psoriasis. J Am Acad Dermatol. 2022;86:148-157.
- [CrossRef] [PubMed] [Google Scholar]
- Novel therapies in plaque psoriasis: a review of tyrosine kinase 2 inhibitors. Dermatol Ther (Heidelb). 2023;13:417-35.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Tyk2 is a therapeutic target for psoriasis-like skin inflammation. Int Immunol. 2014;26:257-67.
- [CrossRef] [PubMed] [Google Scholar]
- JAK inhibitors for treatment of psoriasis: focus on selective TYK2 inhibitors. Drugs. 2020;80:341-52.
- [CrossRef] [PubMed] [Google Scholar]
- Novel Therapies in Plaque Psoriasis: A Review of Tyrosine Kinase 2 Inhibitors. Dermatol Ther (Heidelb). 2023;13:417-35.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Clinical implications of targeting the JAK-STAT pathway in psoriatic disease: emphasis on the TYK2 pathway. J Cutan Med Surg. 2023;27:3S-24S.
- [CrossRef] [PubMed] [Google Scholar]
- Interpreting indirect treatment comparisons and network meta-analysis for health-care decision making: Report of the ISPOR task force on indirect treatment comparisons good research practices: Part 1. Value Health. 2011;14:417-28.
- [CrossRef] [PubMed] [Google Scholar]
- The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: checklist and explanations. Ann Intern Med. 2015;162:777-84.
- [CrossRef] [PubMed] [Google Scholar]
- Bias in meta-analysis detected by a simple, graphical test. Graphical test is itself biased. BMJ (Clinical research ed.). 1998;316:470-1.
- [Google Scholar]
- Ranking treatments in frequentist network meta-analysis works without resampling methods. BMC Med Res Methodol. 2015;15:58.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Cochrane handbook for systematic reviews of interventions version 6.3 (updated February 2022). 2022. Available from: https://www.ncbi.nlm.nih.gov/pubmed/31643080
- Deucravacitinib versus placebo and apremilast in moderate to severe plaque psoriasis: Efficacy and safety results from the 52-week, randomized, double-blinded, phase 3 Program for evaluation of TYK2 inhibitor psoriasis second trial. J Am Acad Dermatol. 2023;88:40-51.
- [CrossRef] [PubMed] [Google Scholar]
- Deucravacitinib versus placebo and apremilast in moderate to severe plaque psoriasis: Efficacy and safety results from the 52-week, randomized, double-blinded, placebo-controlled phase 3 POETYK PSO- 1 trial. J Am Acad Dermatol. 2023;88:29-39.
- [CrossRef] [PubMed] [Google Scholar]
- TYK2/JAK1 inhibitor PF-06700841 in patients with plaque psoriasis: Phase IIa, randomized, double-blind, placebo-controlled trial. J Invest Dermatol. 2020;140:2359-70.
- [CrossRef] [PubMed] [Google Scholar]
- Oral tyrosine kinase 2 inhibitor PF-06826647 demonstrates efficacy and an acceptable safety profile in participants with moderate-to-severe plaque psoriasis in a phase 2b, randomized, double-blind, placebo-controlled study. J Am Acad Dermatol. 2022;87:333-42.
- [CrossRef] [PubMed] [Google Scholar]
- The efficacy and safety of tofacitinib in Asian patients with moderate to severe chronic plaque psoriasis: A Phase 3, randomized, double-blind, placebo-controlled study. J Dermatol Sci. 2017;88:36-45.
- [CrossRef] [PubMed] [Google Scholar]
- Tofacitinib versus etanercept or placebo in moderate-to-severe chronic plaque psoriasis: a phase 3 randomised non-inferiority trial. Lancet (London, England). 2015;386:552-61.
- [CrossRef] [PubMed] [Google Scholar]
- Tofacitinib improves pruritus and health-related quality of life up to 52 weeks: Results from 2 randomized phase III trials in patients with moderate to severe plaque psoriasis. J Am Acad Dermatol. 2016;75:1162-70.
- [CrossRef] [PubMed] [Google Scholar]
- A dose ranging study to evaluate the safety and efficacy of GSK2586184 in patients with chronic plaque psoriasis. (Available from: https://clinicaltrials.gov/ct2/show/NCT01782664 Accessed November 18th 2023)
- A phase 2b study of baricitinib in participants with moderate to severe psoriasis. (Available from: https://clinicaltrials.gov/ct2/show/NCT01490632 Accessed November 18th 2023)
- An investigational study to evaluate experimental medication BMS-986165 compared to placebo in participants with plaque psoriasis (POETYK-PSO-3) in Mainland China , Taiwan, and South Korea. 2019. (Available from: https://www.cochranelibrary.com/central/doi/10.1002/central/CN-02004055/full)
- Phase 2 trial of selective tyrosine kinase 2 inhibition in psoriasis. N Engl J Med. 2018;379:1313-21.
- [CrossRef] [PubMed] [Google Scholar]
- Tofacitinib attenuates pathologic immune pathways in patients with psoriasis: A randomized phase 2 study. J Allergy Clin Immunol. 2016;137:1079-90.
- [CrossRef] [PubMed] [Google Scholar]
- Efficacy and safety of tofacitinib, an oral Janus kinase inhibitor, in the treatment of psoriasis: A Phase 2b randomized placebo-controlled dose-ranging study. Br J Dermatol. 2012;167:668-77.
- [CrossRef] [PubMed] [Google Scholar]
- Apremilast, an oral phosphodiesterase 4 (PDE4) inhibitor, in patients with moderate to severe plaque psoriasis: Results of a phase III, randomized, controlled trial (efficacy and safety trial evaluating the effects of apremilast in psoriasis [ESTEEM] 1) J Am Acad Dermatol. 2015;73:37-49.
- [CrossRef] [PubMed] [Google Scholar]
- Efficacy of apremilast in the treatment of moderate to severe psoriasis: A randomised controlled trial. Lancet (London, England). 2012;380:738-46.
- [CrossRef] [PubMed] [Google Scholar]
- Methotrexate versus cyclosporine in moderate-to-severe chronic plaque psoriasis. N Engl J Med. 2003;349:658-65.
- [CrossRef] [PubMed] [Google Scholar]
- Efficacy and safety results from the randomized controlled comparative study of adalimumab vs. methotrexate vs. placebo in patients with psoriasis (CHAMPION) Br J Dermatol. 2008;158:558-66.
- [CrossRef] [PubMed] [Google Scholar]
- Fumarates vs. methotrexate in moderate to severe chronic plaque psoriasis: A multicentre prospective randomized controlled clinical trial. Br J Dermatol. 2011;164:855-61.
- [CrossRef] [PubMed] [Google Scholar]
- Oral small-molecule tyrosine kinase 2 and phosphodiesterase 4 inhibitors in plaque psoriasis: A network meta-analysis. Front Immunol. 2023;14:1180170.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Tyrosine kinase 2 and Janus kinase-signal transducer and activator of transcription signaling and inhibition in plaque psoriasis. J Am Acad Dermatol. 2022;86:148-57.
- [CrossRef] [PubMed] [Google Scholar]
- Deucravacitinib versus placebo and apremilast in moderate to severe plaque psoriasis: Efficacy and safety results from the 52-week, randomized, double-blinded, placebo-controlled phase 3 POETYK PSO- 1 trial. J Am Acad Dermatol. 2023;88:29-39.
- [CrossRef] [PubMed] [Google Scholar]
- Deucravacitinib versus placebo and apremilast in moderate to severe plaque psoriasis: Efficacy and safety results from the 52-week, randomized, double-blinded, phase 3 Program for Evaluation of TYK2 inhibitor psoriasis second trial. J Am Acad Dermatol. 2023;88:40-51.
- [CrossRef] [PubMed] [Google Scholar]
- Deucravacitinib in the treatment of psoriasis. J Dermatolog Treat. 2023;34:2154122.
- [CrossRef] [PubMed] [Google Scholar]
- Deucravacitinib (Sotyktu™) for plaque psoriasis. Trends Pharmacol Sci. 2023;44:252-3.
- [CrossRef] [PubMed] [Google Scholar]
- Deucravacitinib: First Approval. Drugs. 2022;82:1671-9.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- The JAK/STAT signaling pathway: From bench to clinic. Signal Transduct Target Ther. 2021;6:402.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Joint AAD-NPF guidelines of care for the management and treatment of psoriasis with biologics. J Am Acad Dermatol. 2019;80:1029-72.
- [CrossRef] [PubMed] [Google Scholar]
- Deucravacitinib (Sotyktu): CADTH Reimbursement recommendation. Ottawa (ON): Canadian Agency for Drugs and Technologies in Health; 2023.






