Translate this page into:
Comparison of effectiveness of interventions in reducing mortality in patients of toxic epidermal necrolysis: A network meta-analysis
Corresponding author: Dr. Tejas K. Patel, Department of Pharmacology, All India Institute of Medical Sciences, Gorakhpur 273 008, Uttar Pradesh, India. dr.tkp2006@yahoo.co.in
-
Received: ,
Accepted: ,
How to cite this article: Patel TK, Patel PB, Thakkar S. Comparison of effectiveness of interventions in reducing mortality in patients of toxic epidermal necrolysis: A network meta-analysis. Indian J Dermatol Venereol Leprol 2021;87:628-44.
Abstract
Background:
Limited evidence is available about effectiveness and choice of immunomodulating treatment modalities for toxic epidermal necrolysis (TEN).
Aims:
To compare the effectiveness of interventions to reduce mortality in patients of toxic epidermal necrolysis through network meta-analysis.
Methods:
Studies were retrieved using PubMed, Google Scholar and Cochrane Database of Systematic Reviews from inception to September 18, 2018. Only English language articles were considered. Observational and randomized controlled studies having ≥ 5 TEN patients in each intervention arm were included. Two investigators independently extracted study characteristics, intervention details and mortality data. Bayesian network meta-analysis was performed using the Markov chain Monte Carlo (MCMC) approach through the random effect model. The ranking analysis was done to provide a hierarchy of interventions. The consistency between direct and indirect evidence was assessed through node spit analysis. The primary outcome was to compare the mortality [Odds ratio OR (95% credibility interval CrI)] among all treatment modalities of TEN.
Results:
Twenty-four studies satisfying the selection criteria were included. The network analysis showed improved survival with cyclosporine as compared to supportive care [OR- 0.19 (95% CrI: 0.05, 0.59)] and intravenous immunoglobulin [OR- 0.21 (95% CrI: 0.05, 0.76)]. The hierarchy of treatments based on “surface under the cumulative ranking curves” (SUCRA) value were cyclosporine (0.93), steroid+intravenous immunoglobulin (0.76), etanercept (0.59), steroids (0.46), intravenous immunoglobulin (0.40), supportive care (0.34) and thalidomide (0.02). No inconsistencies between direct and indirect estimates were observed for any of the treatment pairs.
Limitations:
Evidence is mainly based on retrospective studies.
Conclusion:
The use of cyclosporine can reduce mortality in TEN patients. Other promising immunomodulators could be steroid+intravenous immunoglobulin combination and etanercept.
Keywords
Cyclosporine
immunologic factors
mortality
Stevens–Johnson syndrome
Plain language summary
Toxic epidermal necrolysis is a type of severe skin reaction most commonly caused by drugs. It affects almost 1 to 2 million people per year. It is considered an emergency and causes death in 15%–30% of the affected patients. There are no proven effective medications against it. The patients are managed symptomatically in intensive care units. The authors have conducted “network meta-analysis” to find out which is the most effective medication against this skin reaction.
The “network meta-analysis” is a statistical tool to compare data of multiple medications simultaneously from the already published literature. It also provides hierarchies among the medications and identifies the best possible medications against the disease or condition being studied. A total of 24 published studies were analyzed and five medications were compared with each other. The medications were corticosteroid, intravenous immunoglobulin, combination of steroid+intravenous immunoglobulin, etanercept and cyclosporine. The authors found that use of cyclosporine can reduce death due to toxic epidermal necrolysis. The other effective medications could be combination of steroid+intravenous immunoglobulin and etanercept.
Introduction
Stevens–Johnson syndrome (SJS) is considered to be a rare and serious cutaneous reaction. The main causative factor is drugs. It is classified into three categories based on the percentage of body surface area involvement: SJS (<10%), toxic epidermal necrolysis – TEN (>30%) and SJS-TEN overlap (10%–30%).1 They are associated with high morbidity and mortality. An earlier systematic review suggests that TEN is associated with significantly higher mortality than SJS [odd ratio- OR: 7.2 (95% CI: 1.6–31.9)].2 The reported mortality rate of SJS, SJS-TEN overlap and TEN varies from 1.9 to 4.8, 5.3 to 19.4 and 14.3 to 28.2, respectively.2-4
Earlier systematic reviews did not suggest significant survival benefit of steroids,5,6 intravenous immunoglobulin5,7,8 and combination of steroid+intravenous immunoglobulin in SJS/TEN patients.9 A recent individual patient-level meta-analysis suggests steroids and cyclosporine are two most promising immunomodulating treatment options for SJS/ TEN patients.10 Two more recent meta-analyses observed cyclosporine therapy can reduce the risk of mortality in SJS/ TEN patients.11,12 All these earlier meta-analyses had limited direct head to head comparison of treatment modalities.
Unlike traditional meta-analyses, network meta-analysis provides a comparative treatment effectiveness through analysis of both direct and indirect evidence. It also provides hierarchies among the treatment modalities and offers a comprehensive framework for decision-making.13
In this study, we focused on TEN cases with body surface area > 10%. SJS usually have lower mortality than TEN.1-4 We anticipated the inclusion of observational studies. The differences in the number of patients of SJS or TEN in different treatment arms could have affected direct/indirect comparisons and ranking analysis. We conducted the network meta-analysis to compare the effectiveness of interventions to reduce mortality in patients of TEN.
Methods
Information sources and search strategy
Two investigators (TKP and PBP) independently searched the PubMed, Google Scholar and Cochrane Database of Systematic Reviews. We also searched the bibliographies of relevant articles and systematic reviews. There was no restriction on time period to be considered. The search strategy of PubMed and Google Scholar were: (Stevens-Johnson syndrome OR Toxic epidermal necrolysis OR Lyell’s syndrome) AND (Treatment OR Management OR Supportive care OR Palliative care OR Corticosteroid OR Immunoglobulin OR Cyclosporine). We included English language articles only. The last search was carried out on September 9, 2018 on PubMed and September 18, 2018 on Google Scholar. The study protocol was prospectively registered on PROSPERO register (CRD42018092567).
Case definition of TEN
SJS/TEN overlap and TEN were considered as TEN as defined by Bastuji-Garin et al. (body surface area involvement – body surface area >10%).1 In case of absence of apparent classification in the study, raw data of body surface area was used to categorize patients into TEN.
Selection criteria
Inclusion criteria
Observational and randomized controlled studies of any age group assessing the effectiveness of two interventions for reducing mortality in TEN patients. The intervention can be supportive care or any treatment modality
Studies should have ≥ 5 TEN patients in each intervention arm.
Exclusion criteria
Studies not differentiating SJS from TEN or not providing the raw data of body surface area involvement to categorize TEN
SJS/TEN studies not focusing on mortality as an outcome
Non-comparative studies
Duplicate studies (In case of duplicate reports, studies with most comprehensive, up-to-date and largest dataset was included)
Review articles, editorials, non-research letters, discussion papers.
Study screening and selection strategy
Two investigators (TKP and PBP) independently initially assessed title, abstract and then, if potentially relevant, retrieved full text as per selection criteria. All full-text articles were initially screened for differentiation between SJS and TEN based on body surface area, treatment arms, number of included patients in each arm and mortality data. A predefined Excel sheet was used to record the reason of each excluded study. The disagreements in study selection were resolved through discussion, consensus and consultation with third investigator (ST).
Data extraction process
The following data were collected from the included studies in a predefined Excel sheet:
General study characteristics: first author, publication year, types of publication, country, data collection period, study duration, study design, age group studied, admission ward, diagnosis of TEN
Intervention characteristics: Dose, route, duration of each treatment modality studied; basis of assigning treatment; mean or median age, body surface area and SCORTEN score involvement and delay of stating treatment in each treatment arms; observed and expected mortality
Mortality data: treatment sample size and number of patients died in each treatment arm.
All extracted data were cross-checked to ensure accuracy.
Risk of bias (quality) assessment
The risk of bias was assessed using scoring tool designed by Zimmermann et al. for the SJS/TEN studies.10 It scores each study based on clear description of hypothesis, main outcomes, selection criteria, ineligible and those refuse to participate in the study, participants completing the treatment, distributions of the principal confounders (age, severity, country, year) and use of 95%-confidence interval (CI) and/ or actual probability values to report mortality. The range of total score is 0 to 13.17. The score below 5 was used as a cut off point to define high-risk studies.
Statistical analysis
The primary outcome was to compare the mortality among the all treatment modalities of TEN.
Initially, proportions of deaths were analyzed and expressed as Odds ratio (OR) and its 95% CI for each study. The direct pairwise meta-analysis of all interventions was performed using Mantel-Haenszel’s method with random-effect models to evaluate statistical heterogeneity within each comparison. An I2 test was used to evaluate the heterogeneity. An I2 value of 25%, 50% and 75% was considered as low, medium and high heterogeneity, respectively.14
On completion of pairwise meta-analysis, Bayesian network meta-analysis was performed using the Markov chain Monte Carlo (MCMC) approach.15,16 The vague prior distribution was used to obtain the closest findings with frequentist method.17 The pooled OR and its corresponding 95% credibility interval (CrI) was obtained through random effect model for each treatment pair comparison. The treatment modality was considered effective in reducing mortality, when the upper and lower 95% CrI for OR were less than 0 (equivalent to P < 0.05). Network diagram was plotted to depict the treatment modalities that directly compared with each other.
Ranking analysis was done to rank all interventions. Surface under the cumulative ranking curves (SUCRA), a numerical summary of the probabilities, was used to provide a hierarchy of interventions. SUCRA value 100% indicates a treatment is certain to be the best and 0% value suggests a treatment is certain to be the worst.18 Based on SUCRA, the league table was arranged to present the network meta-analysis summary estimates. The treatments were ranked in order of better to worst outcome from left to right in a league table.
The sensitivity analysis of network meta-analysis was performed by risk of bias assessment (excluding the high-risk studies), study design and study region (developed/ developing countries). A comparison-adjusted funnel plot was used to assess publication bias.
Assessment of inconsistency
Node splitting was used to assess consistency between direct and indirect evidence. The mean treatment effect estimates were calculated based on the direct and indirect evidence. The consistency of the estimates of treatment effects was examined to evaluate the discrepancy between direct and indirect comparisons.19
Statistical packages used
The direct pairwise meta-analysis was done through “Review manager software version 5.3.” The network meta-analysis was performed using the Microsoft-Excel-based Network Meta-analysis tool - NetMetaXL version 1.6.1 (Cornerstone research group, Canada) and WinBUGS 1.4.3. software (MRC Biostatistics Unit, Cambridge Institute of Public Health, United Kingdom). The node split analysis was done through MetaInsight (binary) software version 1.1 (Complex review support unit, University of Glasgow, United Kingdom).
Results
Literature search
We assessed 273 full texts and included 24 articles fulfilling the selection criteria from the literature search [Figure 1].
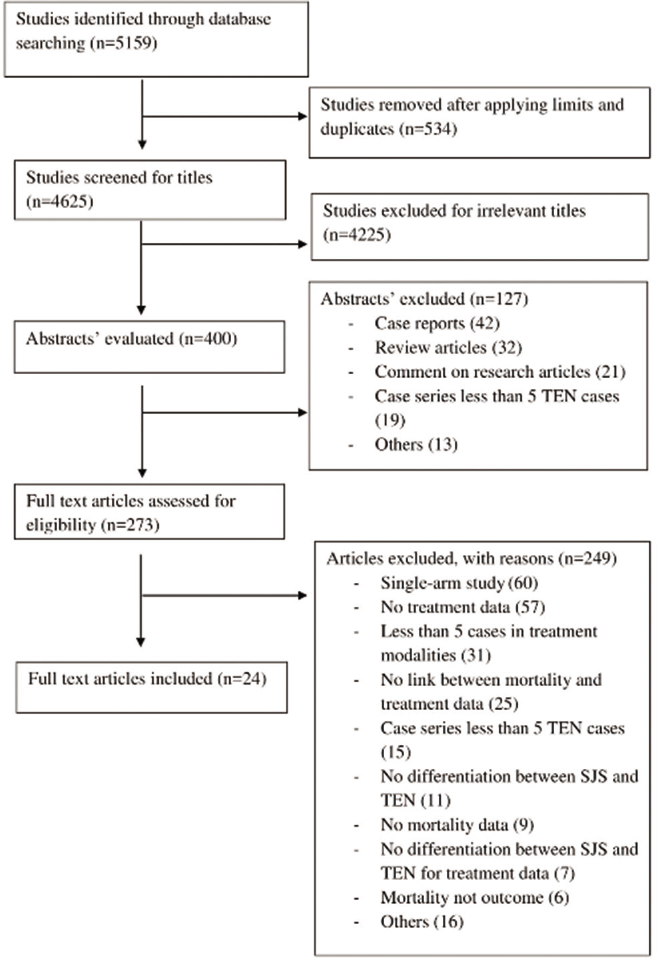
- PRISMA flow diagram showing study selection process
Characteristics of the included studies
The detailed characteristics of all included studies are presented in Table 1.20-43 The study designs of included studies were retrospective (18), prospective (3), randomized controlled trial (2) and prospective-retrospective (1). Ten, twenty and thirty percent body surface area involvement was considered as TEN in 14, 2 and 8 studies, respectively. Twelve studies included all age group and 8 studies adults and elderly age group patients, while four studies did not have a clear description of age group studied. Total sample size of included TEN patients varied from 11 to 174.
| Study | Country | Data collection period | Study design | Study specific department | TEN definition - Percentage of BSA involvement | Diagnosis of TEN | Study age group | Study population | Total TEN sample | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Age to range, mean ± SD* (years) | BSA to range, mean ± SD*, mean (95% CI)**, median (IQR)*** % | SCORTEN score to mean ± SD*, mean (95% CI)** | |||||||||
|
Brand and Rohr, 200020 |
Australia |
1978–1998 |
Retrospective |
ICU |
>30 |
Clinical |
Adults and elderly |
23–73 |
NM |
NM |
12 |
|
Brown et al., 200421 |
USA |
1997–2002 |
Retrospective |
BU |
>10 |
Clinical, biopsy |
NM |
45 ± 25* |
45.6 ± 25* |
NM |
45 |
|
Chantaphakul et al., 201522 |
Thailand |
2009–2014 |
Retrospective |
NM |
>10 |
Clinical |
Adults and elderly |
20–85 |
NM |
NM |
19 |
|
Chen et al., 201023 |
China |
1994–2009 |
Retrospective |
NM |
>30 |
Clinical, biopsy |
All age |
11–81 |
30.2* |
NM |
30 |
|
González-Herrada et al., 201724 |
Spain |
2001–2015 |
Prospective- retrospective |
BU |
>10 |
Clinical, biopsy |
Adults and elderly |
NM |
NM |
NM |
32 |
|
Gravante et al., 200725 |
Italy |
1995–2005 |
Retrospective |
BU |
>10 |
Clinical, biopsy |
All age |
4–94 |
62.8 ± 32.8* |
NM |
31 |
|
Hirapara et al., 201726 |
India |
2009–2012 |
Retrospective |
NM |
>10 |
Clinical |
All age |
6–78 |
38.4 (32.2–44.4)** |
1.8 (1.5–2.0)** |
36 |
|
Ioannides et al., 199427 |
Greece |
1972–1990 |
Retrospective |
NM |
>20 |
Clinical, biopsy |
All age |
2–84 |
42.1 ± 16.1* |
NM |
19 |
|
Jagadeesan et al., 201328 |
India |
2008–2012 |
Prospective |
DW |
>30 |
Clinical ± biopsy |
All age |
6–68 |
51.6* |
NM |
36 |
|
Kaur et al., 199029 |
India |
1982–1989 |
Prospective |
DW |
>20 |
Clinical, biopsy |
All age |
0.4–60 |
23* |
NM |
30 |
|
Kim et al., 200530 |
South Korea |
1990–2003 |
Retrospective |
NM |
>30 |
Clinical, biopsy |
All age |
2–80 |
48.7 ± 17.1* |
NM |
38 |
|
Lalosevic et al., 201531 |
Serbia |
1993–2012 |
Retrospective |
NM |
>30 |
Clinical, biopsy |
All age |
1–94 |
74.0 ± 20.8* |
NM |
17 |
|
Lee et al., 201732 |
Singapore |
2011–2014 |
Retrospective |
BU |
>10 |
Clinical, biopsy |
Adults and elderly |
57 ± 20* |
29 ± 25* |
NM |
28 |
|
Mohanty et al., 201733 |
India |
2014–2015 |
Retrospective |
DW |
>10 |
Clinical |
All age |
38.4 ± 8.8* |
34.9 ± 19.9 |
2.57 ± 1.1 |
22 |
|
Paquet et al., 200634 |
Belgium |
NM |
Prospective |
NM |
>30 |
Clinical, biopsy |
Adults and elderly |
18–78 |
59.0 ± 15.9 |
NM |
11 |
|
Poizeau et al., 201835 |
France |
2005–2016 |
Retrospective |
BU |
>10 |
Clinical |
NM |
NM |
NM |
NM |
174 |
|
Schneck et al., 200836 |
Germany, France |
1997–2001 |
Retrospective |
NM |
>10 |
Clinical ± biopsy |
NM |
47 ± 25* |
NM |
NM |
171 |
|
Shortt et al., 200437 |
Canada |
1995–2002 |
Retrospective |
BU |
>10 |
Clinical ± biopsy |
NM |
NM |
NM |
NM |
32 |
|
Stella et al., 200738 |
Italy |
1993–2005 |
Retrospective |
BU |
>10 |
Clinical, biopsy |
Adults and elderly |
27–81 |
33.3 ± 26.3* |
NM |
27 |
|
Wang et al., 201839 |
Taiwan |
2009–2015 |
RCT Open labeled |
NM |
>10 |
Clinical ± biopsy |
All age |
6–87 |
44.6 ± 23.1* |
NM |
35 |
|
Wolkenstein et al., 199840 |
France |
1995–1996 |
RCT Double blind |
BU, ICU |
>10 |
Clinical, biopsy |
Adults and elderly |
23–81 |
NM |
NM |
22 |
|
Yang et al., 200941 |
China |
1993–2007 |
Retrospective |
ICU |
>10 |
Clinical, biopsy |
All age |
6–86 |
41.0 ± 12.5* |
2.3 ± 1.0 |
47 |
|
Yeong et al., 201142 |
Taiwan |
2000–2006 |
Retrospective |
BU |
>30 |
Clinical, biopsy |
All age |
11–89 |
66.2 ± 28.6 |
3.2 ± 1.4 |
16 |
|
Zhu et al., 201243 |
China |
2000–2010 |
Retrospective |
ICU |
>30 |
Clinical, biopsy |
Adults and elderly |
18–91 |
90.3 ± 12.1 |
2.3 ± 1.2 |
55 |
ICU: Intensive care unit, BU: Burn unit, DW: Dermatology ward, RCT: Randomized controlled trial, NM: Not mentioned, IQR: Interquartile range, TEN: Toxic epidermal necrolysis, SCORTEN: Score of TEN, SD: Standard deviation
Intervention group characteristics
Total of 979 patients with TEN from 24 studies were assigned to 7 intervention groups. Total of 223 deaths were observed. The interventions used in included studies were supportive care (15), steroids (14), intravenous immunoglobulin (8), Steroid+intravenous immunoglobulin (7), Cyclosporine (4), Etanercept (1) and Thalidomide (1). Number of two-arm intervention studies were 23. One study assessed multi-arm interventions.36 Basis of allocation of the treatment was clearly described in 14 studies. Only 5 studies described the delay in start of treatment.21,25,32,35,36 In case of Shortt et al., intravenous immunoglobulin group of patients were admitted significantly earlier than those who received supportive care only.37 The studies that described or provided sufficient data to calculate the age group, body surface area and SCORTEN score distribution of intervention groups were 20, 19 and 9, respectively. In case of Hirapara et al.26 and Lee et al.32 age group data were not comparable among the intervention groups. In case of Stella et al., patients in corticosteroid group had significantly higher body surface area involvement than steroid+intravenous immunoglobulin group patients. In case of Mohanty et al., patients in supportive care group had higher SCORTEN score than cyclosporine group patients.33 Five included studies did not differentiate the mortality data between SJS and TEN patients. The corresponding authors provided the mortality data on request through mail.24,32,33,35,36 Detailed characteristics of intervention groups are presented in Table 2.
| Study | Intervention | Intervention dose | Basis of assigning treatment | Delay in start treatment Mean±SD, mean (95% CI)*, median (IQR)** days |
Age Mean±SD, mean (95% CI)*, median (IQR)**, median (range)*** years |
BSA Mean±SD, median (range)*** % |
SCORTEN score Mean (95% CI)*, median (IQR)**, median (range)*** |
Total TEN sample | Expected mortality percentage | Observed mortality percentage |
|---|---|---|---|---|---|---|---|---|---|---|
| Cyclosporine versus supportive care | ||||||||||
| Lee et al., 201732 | Supportive care | NA | All consecutively admitted patients based on selection criteria received cyclosporine. Rest received supportive care only | 4.0±2.7 | 66±17 | 32±30 | NM | 12 | 29.5 | 30 |
| Cyclosporine | 3 mg/kg/day for 10 days than 2 mg/kg/day for 10 days and lastly 1 mg/kg/day for 10 days | - | 1.8±1.7 | 50±21 | 26±20 | NM | 16 | 29.9 | 12.5 | |
| Mohanty et al., 201733 | Supportive care | NA | All consecutively admitted patients based on selection criteria received cyclosporine. Rest received supportive care only | NM | 41.8±9.6 | 32.8±20.0 | 3.7±1.0 | 8 | 52.5 | 55.5 |
| Cyclosporine | 5 mg/kg/day for 10 days | - | NM | 36.8±8.3 | 35.9±20.3 | 2.05±1.1 | 14 | 16.4 | 5.26 | |
| Poizeau et al., 201835 | Supportive care | NA | NM | ≤3 days | 48 (39–63)** | 10±14 (epidermal detachment) | NM | 79 | NM | NM |
| Cyclosporine | 3 mg/kg/day for 10 days | - | ≤ 3 days | 39 (2–57)** | 18±18 (epidermal detachment) | NM | 95 | NM | NM | |
| Steroid versus supportive care | ||||||||||
| Brand and Rohr, 200020 | Supportive care | NA | Dermatologist discretion | NM | NM | NM | NM | 6 | NM | NM |
| Corticosteroid | HS: 400 mg or PS: 30–100 mg for 3–14 days | - | NM | NM | NM | NM | 6 | NM | NM | |
| Chantaphaku et al., 201522 | Supportive care | NA | NM | NM | NM | NM | NM | 5 | NM | NM |
| Corticosteroid | DS: IV 8–40 mg/day or PS: 30–60 mg/day for 1–10 days | NM | NM | NM | NM | NM | 14 | NM | NM | |
| Hirapara et al., 201726 | Supportive care | NA | NM | NM | 49 (40.–57.6)* | NM | 1.8 (1.3–2.3)*,^ | 8 | NM | NM |
| Corticosteroid | Mean dose- DS: 5.6 mg or HS: 200 mg or PS: 17.5 mg. Mean duration 6.9 days | - | SJS/TEN overlap: 4.4 (3.3–5.4)* TEN: 7.1 (3.3–10.8)* |
34.8 (27.4–42.2)*,^ | NM | 1.8 (1.5–2.1)*,^ | 28 | NM | NM | |
| Ioannides et al., 199427 | Supportive care | NA | NM | NM | 38.7±26.3 | 35.0±13.6 | NM | 8 | NM | NM |
| Corticosteroid | PS equivalent 50–120 mg/day doses of steroids | NM | NM | 50.6±26.2 | 47.3±16.3 | NM | 11 | NM | NM | |
| Kaur et al., 199029 | Supportive care | NA | NM | NM | NM | NM | NM | 9 | NM | NM |
| Corticosteroid | PS: 1–2 mg/kg in a short course | - | NM | NM | NM | NM | 21 | NM | NM | |
| Schneck et al., 200836 | Supportive care | NA | Most patient in Germany received corticosteroid and in France supportive care) | NM | NM | NM | 54 | NM | NM | |
| Corticosteroid | PS equivalent median total dose 250(IQR: 100–500) mg | - | 4 (2–5)** | NM | NM | NM | 62 | NM | NM | |
| IVIG versus supportive care | ||||||||||
| Brown et al., 200421 | Supportive care | NA | NM | 5.6±4.7 | 43±29 | 46.3±26 | 21 | NM | NM | |
| IVIG | 0.4 g/kg/day for 4 days | NM | 9.2±12 | 47±21 | 44.9±24.6 | NM | 24 | NM | NM | |
| Gravante et al., 200725 | Supportive care | NA | Admission years | 11.2±13.9 | 43.7±22.9 | 58.9±34.3 | NM | 15 | NM | NM |
| IVIG | 0.4 g/kg/day for 5 days | 8.9±7.1 | 46.1±18.7 | 66.5±32.1 | NM | 16 | NM | NM | ||
| Paquet et al., 200634 | Supportive care | NA | NM | NM | 44.8±18.6 | 54.8±19.9 | NM | 5 | NM | NM |
| IVIG | 1 g/kg/day for 3 days | - | NM | 46.7±19.2 | 62.5±12.5 | NM | 6 | NM | NM | |
| Schneck et al., 200836 | Supportive care | NA | Most patient in Germany received corticosteroid and in France supportive care) | NM | NM | NM | 54 | NM | NM | |
| IVIG | Median total dose 1.9 g/kg (IQR: 1.3–2.1) | - | 5 (3–7)** | NM | NM | NM | 26 | NM | NM | |
| Shortt et al., 200437 | Supportive care | NA | Historical comparator | 9.1±6.9 | 52±20 | 65±27 | NM | 16 | NM | NM |
| IVIG | 0.2–0.75 g/kg/day for 4 days | - | 4.8±2.6 | 53±21 | 65±29 | NM | 16 | NM | NM | |
| Yeong et al., 201142 | Supportive care | NA | Patients with severe manifestation, uncontrolled progression, sepsis, but not with renal failure received IVIG | NM | 61.7±25.7 | 68.0±28.4 | 3.3±1.2 | 7 | NM | NM |
| IVIG | Doses NM | - | NM | 55.1±23.1 | 64.9±30.4 | 3.1±1.6 | 9 | NM | NM | |
| Steroid+IVIG versus steroid | ||||||||||
| Chen et al., 201023 | Corticosteroid | HS: 100–700 mg/day IV or MPS: 40–80 mg/day. Duration NM | - | NM | 34.7±15.9^ | 15.3^ | 0.8±1.0^ | 15 | NM | NM |
| IVIG+corticosteroid | IVIG: 0.7–7.4 g/kg IV for 3–15 days HS: 100–700 mg/day/methyl prednisolone 40–80 mg/day. Duration NM |
NM | 8.8±4.6 | 42.8±15.1^ | 30.2^ | 2.0±1.7^ | 15 | NM | NM | |
| Jagadeesan et al., 201328 | Corticosteroid | DS: IV 0.1–0.3 mg/kg/day rapidly tapered within 1–2 weeks | Consecutively admitted patients alternatively allocated | NM | 38.6±17.6 | 49.5±14.1 | 2.5 (2–3)** | 18 | 26.4 | 16.7 |
| IVIG+corticosteroid | IVIG: 0.2–0.5 g/kg/day for 3 days DS: Same doses as corticosteroid arm |
- | NM | 35.4±17.7 | 52.8±11.6 | 3 (2–3)** | 18 | 30.5 | 5.55 | |
| Lalosevic et al., 201531 | Corticosteroid | MPS 1–2 mg/kg for 19 mean days | NM | NM | NM | NM | NM | 8 | NM | NM |
| IVIG+corticosteroid | Total 2 g/kg over2 or 5 days | - | NM | NM | NM | NM | 6 | NM | NM | |
| Schneck et al., 200836 | Corticosteroid | PS equivalent median total dose 250(IQR: 100–500) mg | - | 4 (2–5)** | NM | NM | NM | 62 | NM | NM |
| IVIG+corticosteroid | Same doses for corticosteroid and IVIG group | - | NM | NM | NM | NM | 29 | NM | NM | |
| Stella et al., 200738 | Corticosteroid | HS: 200–500 mg MPS: 2 g/BS: 12 mg/day. Duration NM |
Historical comparator | NM | 51.0±16.2 | 71.7±23.8 | NM | 6 | NM | NM |
| IVIG+corticosteroid | IVIG: 0.7 g/kg/day for 4 days | MPS 1 g/day for initial 2 days | NM | 59.3±18.4 | 22.4±13.8 | NM | 21 | NM | NM | |
| Yang et al., 200941 | Corticosteroid | MPS 1–1.5 mg/kg/day till re-epithelialization than prompt tapering | Historical comparator | NM | 43.7±23.1 | 41.3±11.3 | 2.3±1.0 | 35 | 19.2^ | 22.2^ |
| IVIG+corticosteroid | 0.4 g/kg/day for 5days and MPS as mentioned above | NM | 48.2±21.5 | 40.0±16.1 | 2.3±0.9 | 12 | 17.5^ | 15.0^ | ||
| Zhu et al., 201243 | Corticosteroid | MPS 1.5 mg/kg/day till reepithelization than prompt tapering |
Patients with progressive disease after receiving MPS for 3–5days were given IVIG | NM | 51±16 | 84.6±17.8 | 2.4±1.2 | 16 | 24.4 | 22.7 |
| IVIG+corticosteroid | IVIG: 0.4 g/kg/day for 5 days plus MPS as mentioned above | - | NM | 44±20 | 91.7±9.1 | 2.3±1.2 | 39 | 23.9 | 12.8 | |
| IVIG versus steroid | ||||||||||
| Kim et al., 200530 | Corticosteroid | MPS IV 250–1000 mg/day followed by oral PS. Duration NM | NM | NM | NM | NM | NM | 21 | 28.4 | 28.6 |
| IVIG | 1.6–2.0 g/kg. Duration NM | - | NM | NM | NM | NM | 14 | 16.8 | 7.1 | |
| Schneck et al., 200836 | Corticosteroid | PS equivalent median total dose 250(IQR: 100–500) mg | - | 4 (2–5)** | NM | NM | NM | 62 | NM | NM |
| IVIG | Median total dose 1.9 g/kg (IQR: 1.3–2.1) | - | 5 (3–7)** | NM | NM | NM | 26 | NM | NM | |
| Steroid+IVIG versus supportive care | ||||||||||
| Schneck et al.,36 2008 | Supportive care | NA | Most patient in Germany received corticosteroid and in France supportive care) | NM | NM | NM | 54 | NM | NM | |
| IVIG+corticosteroid | Same doses for corticosteroid and IVIG group |
- | NM | NM | NM | NM | 29 | NM | NM | |
| Cyclosporine versus IVIG | ||||||||||
| González-Herrada et al., 201724 | Cyclosporine | 3 mg/kg/day orally or 1 mg/kg/day IV till reepithelization and tapered off | Admission in one of two hospitals | NM | 47.0±17.2^ | 39.3±25.8^ | 2.4±1.1^ | 23 | 25 | 7.7 |
| IVIG | 0.75 g/kg/day for 4 days | - | NM | 55.0±20.8^ | 29.9±26.2^ | 2.7±1.3^ | 9 | 33.6 | 45.5 | |
| Steroid+IVIG versus IVIG | ||||||||||
| Schneck et al., 200836 | IVIG | Median total dose 1.9 g/kg(IQR: 1.3–2.1) |
- | 5 (3–7)** | NM | NM | NM | 26 | NM | NM |
| IVIG+corticosteroid | Same doses for corticosteroid and IVIG group |
- | NM | NM | NM | NM | 29 | NM | NM | |
| Etanercept versus steroid | ||||||||||
| Wang et al., 201839 | Corticosteroid | PS 1–1.5 mg/kg/day IV until skin lesion healed | Randomization with allocation concealment | NM | 57.3±24.4 | 42.1±22.4 | 1.9±1.4^ | 17 | 20.3 | 16.3 |
| Etanercept | 25/50 mg sc twice a week until skin lesion healed | 51.6±15.7 | 46.3±24.2 | 1.8±1.3^ | 18 | 17.7 | 8.3 | |||
| Thalidomide versus supportive care | ||||||||||
| Wolkenstein et al., 199840 | Placebo (supportive care) |
NA | Randomization, double blind using placebo | NM | 50.5 (23–58)*** | 30.5 (10–85)*** | NM | 10 | NM | NM |
| Thalidomide | 400 mg/day for 5 days | 53 (23–81)*** | 43.5 (26–90)*** | NM | 12 | NM | NM | |||
^Data of all SJS patients, TEN: Toxic epidermal necrolysis, SCORTEN: Score of TEapplicable, IV: Intravenous, IVIG: IV immunoglobulin, MPS: Methylprednisolone, HS: Hydrocortisone, PS: Prednisolone, DS: Dexamethasone, BS: Betamethasone, SJS: Stevens–Johnson syndrome
Risk of bias assessment
Total 16 studies scored ≥5 in risk of bias assessment. As shown in risk of bias summary [Figure 2], most of the studies did not clearly describe the details of ineligible participants, eligible subjects refused to participate, patients completed the allocated treatment regimen and years of treatment for each group of patients. The details of risk of bias assessment in individual studies are described in Table 3.
| Study | Description of study hypothesis/aim/objective | Description of main outcome in introduction/methods | Identification of main outcome | Selection criteria description | Ineligible participants’ details | Eligible subjects refused to participate | Patients completed the allocated treatment regimen | Description of following confounding factor distribution for each group of patients | Reporting of 95% CI and/or P value for mortality data* | Total score | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age | Severity | BSA | Years | ||||||||||
| Brand and Rohr, 200020 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 2 |
| Brown et al., 200421 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 0.33 | 4.33 |
| Chantaphakul et al., 201522 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0.33 | 2.33 |
| Chen et al., 201023 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 0 | 0.67 | 4.67 |
| González-Herrada et al., 201724 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 1 | 1 | 1 | 0 | 0.67 | 7.67 |
| Gravante et al., 200725 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 0.33 | 5.33 |
| Hirapara et al., 201726 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 0 | 0.33 | 4.33 |
| Ioannides et al., 199427 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 1 | 1 | 0 | 0 | 5 |
| Jagadeesan et al., 201328 | 1 | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 1 | 1 | 0 | 0.67 | 6.67 |
| Kaur et al., 199029 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| Kim et al., 200530 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0.67 | 4.67 |
| Lalosevic et al., 201531 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0.33 | 2.33 |
| Lee et al., 201732 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 0 | 0.67 | 8.67 |
| Mohanty et al., 201733 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 0.33 | 6.33 |
| Paquet et al., 200634 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 0 | 0 | 6 |
| Poizeau et al., 201835 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 0.67 | 7.67 |
| Schneck et al., 200836 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 0 | 0.67 | 6.67 |
| Shortt et al., 201437 | 1 | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 1 | 1 | 0 | 0.33 | 6.33 |
| Stella et al., 200738 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 0 | 0.67 | 5.67 |
| Wang et al., 201839 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 0 | 0.67 | 9.67 |
| Wolkenstein et al., 199840 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 0.67 | 10.67 |
| Yang et al., 200941 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 0 | 0.67 | 5.67 |
| Yeong et al., 201142 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 1 | 1 | 1 | 0 | 0.33 | 7.33 |
| Zhu et al., 201243 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 0.67 | 8.67 |
No description of item carries score 0, Description of each point score=1 except for reporting of mortality data where description of “95% CI and P value both” carries score - 0.67 and “only P value reporting” carries score - 0.33. BSA: Body surface area, CI: Confidence interval
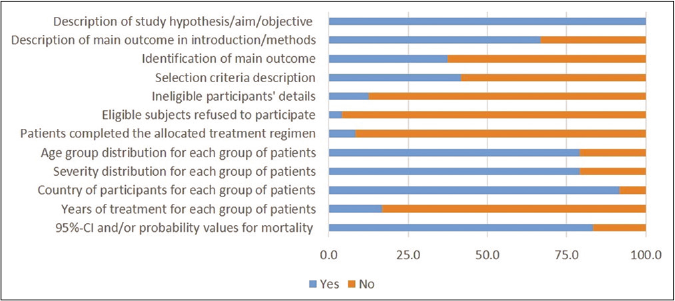
- Risk of bias summary
Direct pairwise meta-analysis
There were total 10 direct pairwise comparisons [Figure 3]. Cyclosporine was associated with significantly reduced risk of mortality as compared with supportive care [OR- 0.32 (95% CI: 0.13, 0.82)] and intravenous immunoglobulin [OR- 0.08 (95% CI: 0.01, 0.54)]. Steroid+intravenous immunoglobulin combination also showed significantly reduced mortality as compared to intravenous immunoglobulin alone [OR- 0.16 (95% CI: 0.04, 0.61)]. Thalidomide was associated with a significantly higher risk of mortality as compared with supportive care [OR-11.67 (95% CI: 1.53, 89.12)]. Other pairwise comparisons did not show a statistically significant difference. There was no significant heterogeneity in a pairwise comparison with an exception of comparison between intravenous immunoglobulin and steroids (I2=72%).
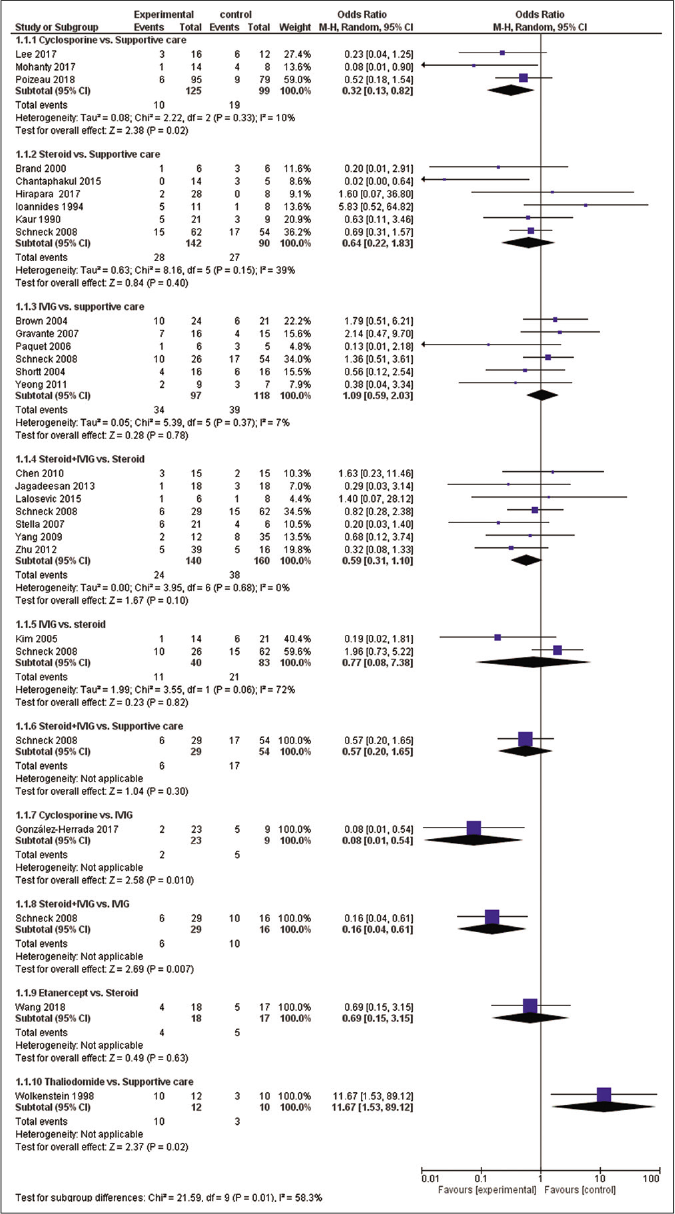
- Meta-analytic summary of direct treatment comparisons
Network meta-analysis
The network of direct treatment comparisons is presented in Figure 4. The size of each node corresponds to the number of participants and thickness of line between the nodes indicate number of comparisons. In line with direct meta-analysis, network analysis showed that risk of death was reduced in cyclosporine arm as compared with supportive care [OR-0.19 (95% CrI: 0.05, 0.59)] and intravenous immunoglobulin [OR- 0.21 (95% CrI: 0.05, 0.76)]. Interventions which showed reduced risk of death as compared to thalidomide were cyclosporine [OR- 0.01 (95% CrI: 0.00 – 0.31)], steroid+intravenous immunoglobulin [OR- 0.03 (95% CrI: 0.00 – 0.51)], steroids [OR- 0.06 (95% CrI: 0.00 – 0.90)] and supportive care [OR- 0.08 (95% CrI: 0.00 – 0.95)]. Unlike direct comparison, Steroid+intravenous immunoglobulin combination did not show significantly reduced mortality as compared to intravenous immunoglobulin alone [OR- 0.45 (95% CrI: 0.13, 1.60)]. Other pairwise comparisons did not show statistically significant difference [Figures 5a and b]. As shown in Table 4, the hierarchy of treatments based on SUCRA value were cyclosporine (0.93), steroid+intravenous immunoglobulin (0.76), etanercept (0.59), steroids (0.46), intravenous immunoglobulin (0.40), supportive care (0.34) and thalidomide (0.02).
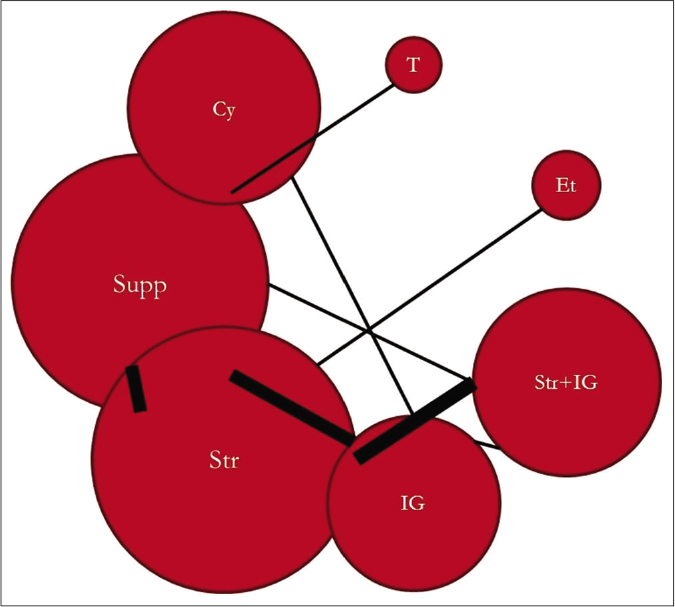
- Network plot of treatment comparison. Cy: Cyclosporine, Supp: Supportive care, Str: Steroid, IG: Intravenous immunoglobulin, Str + IG: Steroid + intravenous immunoglobulin, Et: Etanercept, T: Thalidomide, Size of each node corresponds to number of participants. Thickness of line between nodes indicate number of comparisons
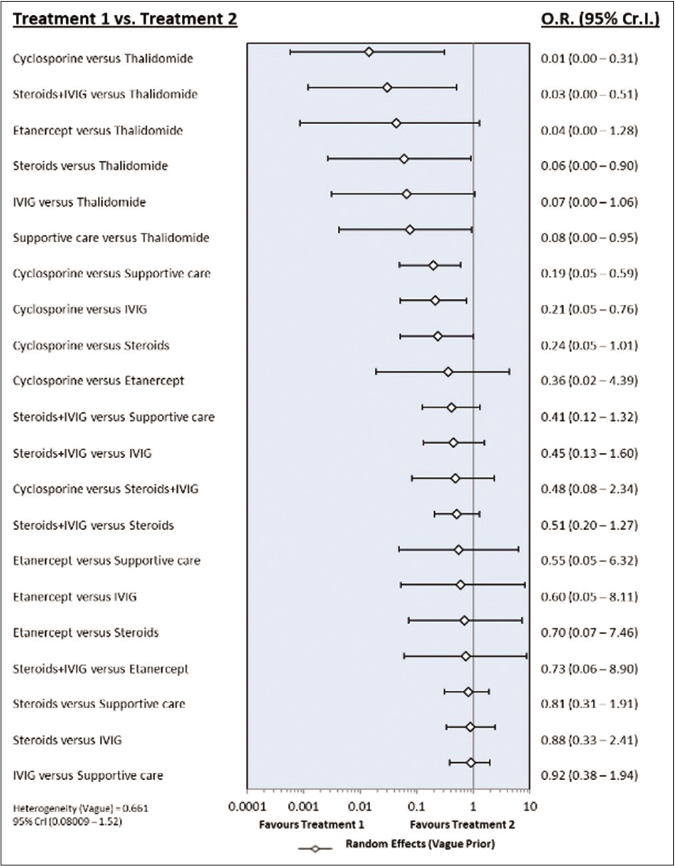
- Forest plot of treatment comparisons for mortality. OR: Odds ratio, CrI: Credibility interval, IVIG: Intravenous immunoglobulin
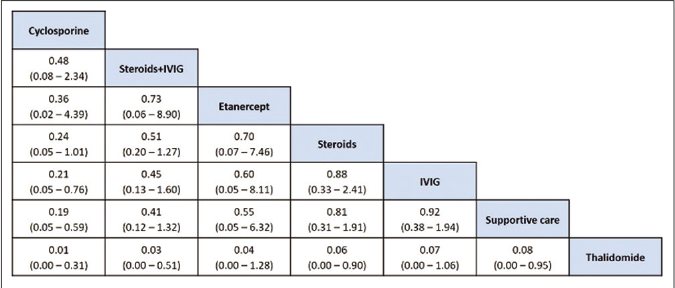
- League table of treatment ranking in order of better to worst outcome from left to right. Data indicates OR: Odds ratio, CrI: Credibility interval, IVIG: Intravenous immunoglobulin
| All studies | Low risk studies (n=16) | Retrospective design (n=18) | Developed countries (n=11) | Developing countries (n=13) |
|---|---|---|---|---|
| Cyclosporine (0.93) | Cyclosporine (0.96) | Cyclosporine (0.90) | Cyclosporine (0.89) | Cyclosporine (0.73) |
| Steroid+IVIG (0.76) | Steroid+IVIG (0.78) | Steroid+IVIG (0.76) | Steroid+IVIG (0.81) | IVIG (0.72) |
| Etanercept (0.59) | Etanercept (0.56) | Steroids (0.38) | Steroids (0.50) | Steroid+IVIG (0.58) |
| Steroids (0.46) | Supportive care (0.42) | IVIG (0.38) | Supportive care (0.46) | Etanercept (0.53) |
| IVIG (0.40) | Steroids (0.40) | Supportive care (0.22) | IVIG (0.32) | Steroids (0.34) |
| Supportive care (0.34) | IVIG (0.38) | - | Thalidomide (0.02) | Supportive care(0.11) |
| Thalidomide (0.02) | Thalidomide (0.01) | - | - | - |
IVIG: Intravenous immunoglobulin
Sensitivity analysis
Risk of bias assessment: The sensitivity analysis was performed by excluding high risk studies. No major differences were observed between all included studies and low-risk bias studies. As shown in Figures 6a and b, the risk of death was significantly reduced with cyclosporine as compared with supportive care, intravenous immunoglobulin and steroids. Interventions that showed a reduced risk of death as compared to thalidomide were cyclosporine, steroid+intravenous immunoglobulin, steroids, intravenous immunoglobulin and supportive care. Steroid+intravenous immunoglobulin combination also showed significantly reduced mortality as compared to intravenous immunoglobulin alone. As shown in Table 4, the most effective interventions based on SUCRA value were cyclosporine (0.96), steroid+intravenous immunoglobulin (0.78) and etanercept (0.56).
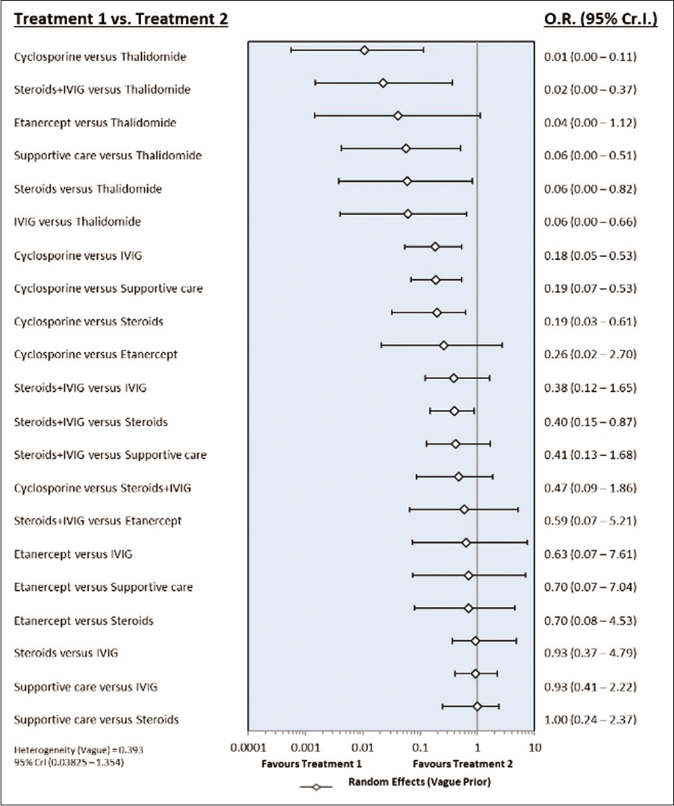
- Forest plot of treatment comparisons for mortality in low risk of bias studies. OR: Odds ratio, CrI: Credibility interval, IVIG: Intravenous immunoglobulin
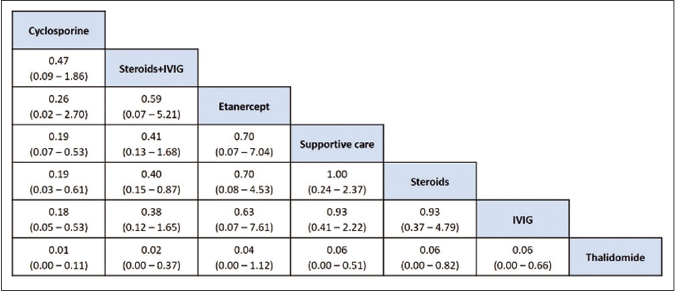
- League table of treatment ranking in order of better to worst outcome from left to right (low risk of bias studies). Data indicates OR: Odds ratio, CrI: Credibility interval, IVIG: Intravenous immunoglobulin
Study design: The sensitivity analysis was performed for retrospective design studies. The risk of mortality was significantly reduced with cyclosporine as compared with supportive care [OR- 0.24 (95% CrI: 0.07, 0.92)]. The most effective treatments hierarchy based on SUCRA value were cyclosporine, steroid+intravenous immunoglobulin and steroids [Table 4]. It was not possible to explore other designs due to the small number of studies in each group [prospective (3), randomized controlled trial (2) and prospective-retrospective (1)].
Study location: The sensitivity analysis was performed based on studies conducted in developed or developing countries. Cyclosporine is the most effective intervention in both developed and developing countries. The other effective treatments in developed countries were steroid+intravenous immunoglobulin and steroids, while in developing countries were intravenous immunoglobulin and steroid+intravenous immunoglobulin [Table 4].
Inconsistency assessment
Both direct and indirect evidence was available for 8 treatment pairs which were part of the closed loop of network meta-analysis. As shown in Tables 5a and b, there were no inconsistencies between direct and indirect estimates of any of the treatment pairs (all 95% CIs across zero and p>0.05) for all studies and low risk of bias studies.
| Comparison | Number of studies | Log_NMA | Log_direct | Log_indirect | Log_difference | Log_diff_95 CI_lower | Log_diff_95 CI_upper | P |
|---|---|---|---|---|---|---|---|---|
| Cyclosporine: IVIG | 1 | −1.46 | −2.57 | −1.10 | −1.48 | −3.92 | 0.97 | 0.24 |
| Cyclosporine: Supportive care | 3 | −1.43 | −1.18 | −2.65 | 1.48 | −0.97 | 3.92 | 0.24 |
| IVIG: Steroids | 2 | 0.25 | 0.15 | 0.36 | −0.21 | −1.87 | 1.46 | 0.81 |
| IVIG: Steroids+IVIG | 1 | 0.83 | 0.87 | 0.79 | 0.08 | −1.89 | 2.05 | 0.94 |
| IVIG: Supportive care | 6 | 0.03 | 0.05 | −0.04 | 0.09 | −1.75 | 1.92 | 0.93 |
| Steroids: Steroids+IVIG | 7 | 0.58 | 0.55 | 1.34 | −0.79 | −4.60 | 3.02 | 0.68 |
| Steroids: Supportive care | 6 | −0.22 | −0.42 | 0.53 | −0.95 | −2.73 | 0.84 | 0.30 |
| Steroids+IVIG: Supportive care | 1 | −0.80 | −0.57 | −0.99 | 0.43 | −1.40 | 2.25 | 0.65 |
NMA: Network meta-analysis, CI: Confidence interval, IVIG: Intravenous immunoglobulin
| Comparison | Number of studies | Log_NMA | Log_direct | Log_indirect | Log_difference | Log_diff_95 CI_lower | Log_diff_95 CI_upper | P |
|---|---|---|---|---|---|---|---|---|
| Cyclosporine: IVIG | 1 | −1.49 | −2.57 | −1.13 | −1.45 | −3.81 | 0.92 | 0.23 |
| Cyclosporine: Supportive care | 3 | −1.36 | −1.13 | −2.58 | 1.45 | −0.92 | 3.81 | 0.23 |
| IVIG: Steroids | 1 | 0.17 | 0.67 | −0.73 | 1.40 | −0.51 | 3.32 | 0.15 |
| IVIG: Steroids+IVIG | 1 | 0.96 | 0.87 | 1.09 | −0.21 | −2.29 | 1.86 | 0.84 |
| IVIG: Supportive care | 5 | 0.13 | −0.07 | 1.82 | −1.89 | −4.15 | 0.36 | 0.10 |
| Steroids: Steroids+IVIG | 5 | 0.79 | 0.73 | 6.68 | −5.94 | −13.64 | 1.74 | 0.13 |
| Steroids: Supportive care | 2 | −0.04 | −0.06 | 0.04 | −0.10 | −2.17 | 1.97 | 0.92 |
| Steroids+IVIG: Supportive care | 1 | −0.83 | −0.57 | −1.25 | 0.68 | −1.26 | 2.63 | 0.49 |
NMA: Network meta-analysis, CI: Confidence interval, IVIG: Intravenous immunoglobulin
The comparison-adjusted funnel plot indicated absence of major asymmetry around zero line [Figure 7].
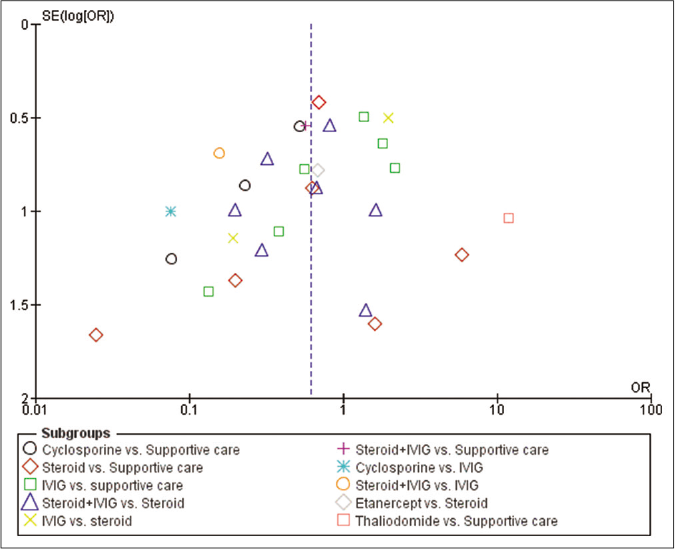
- Funnel plot
Discussion
In this network meta-analysis, we compared the effectiveness of five immunomodulating treatment modalities for TEN patients - steroid, intravenous immnunoglobulin, combination of steroid+intravenous immunoglobulin, etanercept and cyclosporine. Our findings are based on a sample of 979 patients of TEN from 24 studies. Cyclosporine, steroid+intravenous immunoglobulin combination, etanercept, steroid and intravenous immunoglobulin were ranked above supportive care. Probabilities of being a better intervention than supportive care did not alter for cyclosporine, steroid+intravenous immunoglobulin combination and etanercept in sensitivity analysis.
Cyclosporine can decrease the mortality in TEN patients. It showed beneficial effects as compared with supportive care and intravenous immunoglobulin in this study. Cyclosporine was ranked first in SURCRA analysis. This is in line with three earlier meta-analyses suggesting its beneficial effect on patient survival.10-12 Similar survival benefits of cyclosporine were also observed in other studies which are not part of included studies in this network meta-analysis.44-49 Poizeau et al. observed no survival benefit with cyclosporine on propensity score adjustment. However, the authors mentioned that patients with the nonprogressive disease were more likely to have received supportive care than those with cyclosporine.35 Our findings should be interpreted cautiously as they are based on four retrospective studies only. Moreover, included studies either did not consider patients with comorbidities (renal insufficiency, infection, cancer, etc.) or did not report this information.
Ranking analysis suggests etanercept as a promising immunomodulating option for TEN patients. This should be interpreted cautiously as it could not show significant survival benefit over other interventions. The wide confidence interval could be due to only one included study and small sample size. A double-blind randomized control clinical trial has been registered on clinicaltrial.gov (NCT02987257) which is intended to compare cyclospprin, etanercept and supportive care with the sample of 267 patients. Though mortality is the secondary objective, this trial can validate our findings of beneficial effect of cyclosporine and etanercept over supportive care in TEN patients.50
Intravenous immunoglobulin or steroids alone do not improve survival in TEN patients. Both the therapies showed trends of higher mortality than cyclosporine, steroid+intravenous immunoglobulin combination and etanercept. In sensitivity analysis, they showed the trend of worse outcome than supportive care. These findings are in accordance with the earlier meta-analyses.5-8,10 In a meta-analysis by Zimmermann et al., steroid showed significant survival benefit in unstratified individual patient data meta-analysis. However, it was not substantiated on stratified type individual patient data and study level meta-analysis.10 Earlier meta-analyses observed contradictory mortality findings with different doses of intravenous immunoglobulin.7,8,51 We could not evaluate high vs. low dose effect due to small sample of studies in intravenous immunoglobulin group. Similarly, we also could not evaluate the effect of individual steroids, their doses and duration of therapy on mortality.
In contrast to steroids and intravenous immunoglobulin monotherapy, their combination stands second on ranking analysis. It suggests better therapeutic effect with combination than intravenous immunoglobulin or steroids alone. In direct pairwise comparison, seven of nine included studies of combination therapy showed a trend of improved survival than intravenous immunoglobulin, steroid and supportive care alone. Though a meta-analysis by Ye et al. did not observe mortality benefit with steroid+intravenous immunoglobulin combination in SJS/TEN patients, the authors observed significant benefit in TEN patients irrespective of intravenous immunoglobulin dose in combination therapy.9 This is also corroborated by a recent multicentric retrospective study from the United States with a larger sample size which observed the lowest standardized mortality ratio with combination therapy than therapy with intravenous immunoglobulin, steroid or supportive care alone.52
Limitations
This network meta-analysis has several limitations. Only two databases (PubMed and Google Scholar) for English language studies were searched. This could have missed some of the literature. We did not consider SJS (body surface area < 10%) cases. This prevented us from checking the effect of interventions on all severity of SJS cases. We have not included single-arm studies through matching of study characteristics. It could have strengthened the evidence. The network meta-analysis summary and ranking are mainly based on observational studies. Most studies did not describe the method of treatment allocation. There is a possibility that patients could have been treated with a corticosteroid at an outside hospital prior to admission. Most studies did not describe the time-gap between the development of symptoms and initiation of therapy. It could have affected the mortality across the treatment groups. This could also be due to differences in use of supportive care across the studies. Studies also used varied doses and duration of therapy for each intervention.
Conclusion
Cyclosporine reduces the mortality in TEN patients. Other promising interventions could be steroid+intravenous immunoglobulin combination and etanercept. Our findings could be biased as the evidence is based on analysis of retrospective studies. Double-blind randomized studies are recommended to compare the effect of interventions like cyclosporine, steroid+intravenous immunoglobulin and etanercept with supportive care. Investigators planning prospective studies should use a randomized study design. Smaller sample randomized study may not show statistically meaningful mortality differences but will definitely contribute to meta-analysis of randomized controlled studies.
Acknowledgment
We would like to thank following authors who provided further information regarding their articles: Francisco J. de Abajo (González-Herrada et al., 2017),24 Haur Yueh Lee (Lee et al., 2017),32 Nilay Kanti Das (Mohanty et al., 2017),33 Laurence Fardet (Poizeau et al., 2018),35 Peggy Sekula (Schneck et al., 2008).36
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- Clinical classification of cases of toxic epidermal necrolysis, Stevens-Johnson syndrome, and erythema multiforme. Arch Dermatol. 1993;129:92-6.
- [CrossRef] [PubMed] [Google Scholar]
- A systematic review of the drug-induced Stevens-Johnson syndrome and toxic epidermal necrolysis in Indian population. Indian J Dermatol Venereol Leprol. 2013;79:389-98.
- [CrossRef] [PubMed] [Google Scholar]
- Morbidity and mortality of Stevens-Johnson syndrome and toxic epidermal necrolysis in United States adults. J Invest Dermatol. 2016;136:1387-97.
- [CrossRef] [PubMed] [Google Scholar]
- Retrospective analysis of Stevens-Johnson syndrome and toxic epidermal necrolysis in 87 Japanese patients--Treatment and outcome. Allergol Int. 2016;65:74-81.
- [CrossRef] [PubMed] [Google Scholar]
- Systematic review of treatments for Stevens-Johnson syndrome and toxic epidermal necrolysis using the SCORTEN score as a tool for evaluating mortality. Ther Adv Drug Saf. 2011;2:87-94.
- [CrossRef] [PubMed] [Google Scholar]
- Corticosteroids in Stevens-Johnson Syndrome/ toxic epidermal necrolysis: current evidence and implications for future research. Ann Pharmacother. 2015;49:335-42.
- [CrossRef] [PubMed] [Google Scholar]
- The efficacy of intravenous immunoglobulin for the treatment of toxic epidermal necrolysis: A systematic review and meta-analysis. Br J Dermatol. 2012;167:424-32.
- [CrossRef] [PubMed] [Google Scholar]
- Intravenous immunoglobulin for the treatment of toxic epidermal necrolysis: A systematic review and meta-analysis. G Ital Dermatol Venereol. 2016;151:515-24.
- [Google Scholar]
- The effect of intravenous immunoglobulin combined with corticosteroid on the progression of Stevens-Johnson syndrome and toxic epidermal necrolysis: A meta-analysis. PLoS One. 2016;11:e0167120.
- [CrossRef] [PubMed] [Google Scholar]
- Systemic immunomodulating therapies for Stevens-Johnson syndrome and toxic epidermal necrolysis: A systematic review and meta-analysis. JAMA Dermatol. 2017;153:514-22.
- [CrossRef] [PubMed] [Google Scholar]
- Efficacy of cyclosporine for the treatment of Stevens-Johnson syndrome and toxic epidermal necrolysis: Systemic review and meta-analysis. Dermatol Sin. 2017;35:131-7.
- [CrossRef] [Google Scholar]
- A meta-analysis of cyclosporine treatment for Stevens-Johnson syndrome/toxic epidermal necrolysis. J Inflamm Res. 2018;11:135-42.
- [CrossRef] [PubMed] [Google Scholar]
- The attractiveness of network meta-analysis: A comprehensive systematic and narrative review. Heart Lung Vessel. 2015;7:133-42.
- [Google Scholar]
- Measuring inconsistency in meta-analyses. BMJ. 2003;327:557-60.
- [CrossRef] [PubMed] [Google Scholar]
- Borrowing strength from external trials in a meta-analysis. Stat Med. 1996;15:2733-49.
- [CrossRef] [Google Scholar]
- Simultaneous comparison of multiple treatments: Combining direct and indirect evidence. BMJ. 2005;331:897-900.
- [CrossRef] [PubMed] [Google Scholar]
- Methods in health service research, An introduction to bayesian methods in health technology assessment. BMJ. 1999;319:508-12.
- [CrossRef] [PubMed] [Google Scholar]
- Graphical methods and numerical summaries for presenting results from multiple-treatment meta-analysis: An overview and tutorial. J Clin Epidemiol. 2011;64:163-71.
- [CrossRef] [PubMed] [Google Scholar]
- Checking consistency in mixed treatment comparison meta-analysis. Stat Med. 2010;29:932-44.
- [CrossRef] [PubMed] [Google Scholar]
- Toxic epidermal necrolysis in Western Australia. Australas J Dermatol. 2000;41:31-3.
- [CrossRef] [PubMed] [Google Scholar]
- Toxic epidermal necrolysis: Does immunoglobulin make a difference? J Burn Care Rehabil. 2004;25:81-8.
- [CrossRef] [PubMed] [Google Scholar]
- Clinical characteristics and treatment outcome of Stevens-Johnson syndrome and toxic epidermal necrolysis. Exp Ther Med. 2015;10:519-24.
- [CrossRef] [PubMed] [Google Scholar]
- High-dose intravenous immunoglobulins in the treatment of Stevens-Johnson syndrome and toxic epidermal necrolysis in Chinese patients: A retrospective study of 82 cases. Eur J Dermatol. 2010;20:743-7.
- [Google Scholar]
- Cyclosporine use in epidermal necrolysis is associated with an important mortality reduction: Evidence from three different approaches. J Invest Dermatol. 2017;137:2092-100.
- [CrossRef] [PubMed] [Google Scholar]
- Toxic epidermal necrolysis and Steven Johnson syndrome: 11-years experience and outcome. Eur Rev Med Pharmacol Sci. 2007;11:119-27.
- [Google Scholar]
- Drug-induced Stevens-Johnson syndrome in Indian population: A multicentric retrospective analysis. Niger J Clin Pract. 2017;20:978-83.
- [Google Scholar]
- Toxic epidermal necrolysis: A study of 22 cases. J Eur Acad Dermatol Venereol. 1994;3:266-75.
- [CrossRef] [Google Scholar]
- Low dose intravenous immunoglobulins and steroids in toxic epidermal necrolysis: A prospective comparative open-labelled study of 36 cases. Indian J Dermatol Venereol Leprol. 2013;79:506-11.
- [CrossRef] [PubMed] [Google Scholar]
- Elucidation and management of 30 patients of drug induced toxic epidermal necrolysis (DTEN) Indian J Dermatol Venereol Leprol. 1990;56:196-9.
- [Google Scholar]
- Toxic epidermal necrolysis: Analysis of clinical course and SCORTEN-based comparison of mortality rate and treatment modalities in Korean patients. Acta Derm Venereol. 2005;85:497-502.
- [Google Scholar]
- Stevens-Johnson syndrome and toxic epidermal necrolysis: A 20-year single-center experience. Int J Dermatol. 2015;54:978-84.
- [CrossRef] [PubMed] [Google Scholar]
- Cyclosporine treatment for Stevens-Johnson syndrome/toxic epidermal necrolysis: Retrospective analysis of a cohort treated in a specialized referral center. J Am Acad Dermatol. 2017;76:106-13.
- [CrossRef] [PubMed] [Google Scholar]
- Effectiveness, safety and tolerability of cyclosporine versus supportive treatment in Stevens-Johnson Syndrome/Toxic Epidermal Necrolysis: A record-based study. Indian J Dermatol Venereol Leprol. 2017;83:312-6.
- [CrossRef] [PubMed] [Google Scholar]
- Skin immunoglobulin deposition following intravenous immunoglobulin therapy in toxic epidermal necrolysis. Exp Dermatol. 2006;15:381-6.
- [CrossRef] [PubMed] [Google Scholar]
- Cyclosporine for epidermal necrolysis: Absence of Beneficial effect in a retrospective cohort of 174 patients-exposed/unexposed and propensity score-matched analyses. J Invest Dermatol. 2018;138:1293-300.
- [CrossRef] [PubMed] [Google Scholar]
- Effects of treatments on the mortality of Stevens-Johnson syndrome and toxic epidermal necrolysis: A retrospective study on patients included in the prospective EuroSCAR Study. J Am Acad Dermatol. 2008;58:33-40.
- [CrossRef] [PubMed] [Google Scholar]
- Intravenous immunoglobulin does not improve outcome in toxic epidermal necrolysis. J Burn Care Rehabil. 2004;25:246-55.
- [CrossRef] [PubMed] [Google Scholar]
- Toxic epidermal necrolysis (TEN) and Stevens-Johnson syndrome (SJS): experience with high-dose intravenous immunoglobulins and topical conservative approach. A retrospective analysis. Burns. 2007;33:452-9.
- [CrossRef] [PubMed] [Google Scholar]
- Randomized, controlled trial of TNF-α antagonist in CTL-mediated severe cutaneous adverse reactions. J Clin Invest. 2018;128:985-96.
- [CrossRef] [PubMed] [Google Scholar]
- Randomised comparison of thalidomide versus placebo in toxic epidermal necrolysis. Lancet. 1998;352:1586-9.
- [CrossRef] [Google Scholar]
- Combination therapy of intravenous immunoglobulin and corticosteroid in the treatment of toxic epidermal necrolysis and Stevens-Johnson syndrome: A retrospective comparative study in China. Int J Dermatol. 2009;48:1122-8.
- [CrossRef] [PubMed] [Google Scholar]
- Serum bicarbonate as a marker to predict mortality in toxic epidermal necrolysis. J Intensive Care Med. 2011;26:250-4.
- [CrossRef] [PubMed] [Google Scholar]
- Toxic epidermal necrolysis: Performance of SCORTEN and the score-based comparison of the efficacy of corticosteroid therapy and intravenous immunoglobulin combined therapy in China. J Burn Care Res. 2012;33:e295-308.
- [CrossRef] [PubMed] [Google Scholar]
- Open trial of ciclosporin treatment for Stevens-Johnson syndrome and toxic epidermal necrolysis. Br J Dermatol. 2010;163:847-53.
- [CrossRef] [PubMed] [Google Scholar]
- Retrospective review of Stevens-Johnson syndrome/toxic epidermal necrolysis treatment comparing intravenous immunoglobulin with cyclosporine. J Am Acad Dermatol. 2014;71:941-7.
- [CrossRef] [PubMed] [Google Scholar]
- management of toxic epidermal necrolysis with plasmapheresis and cyclosporine A: Our 10 years' experience. Plast Reconstr Surg Glob Open. 2017;5:e1221.
- [CrossRef] [PubMed] [Google Scholar]
- Cyclosporine for SJS/TEN: A case series and review of the literature. Cutis. 2011;87:24-9.
- [Google Scholar]
- Cyclosporine in Stevens Johnson syndrome and toxic epidermal necrolysis and retrospective comparison with systemic corticosteroid. Indian J Dermatol Venereol Leprol. 2013;79:686-92.
- [CrossRef] [PubMed] [Google Scholar]
- Comparison of cyclosporine with systemic corticosteroid in Stevens-Johnson syndrome and toxic epidermal necrolysis-A pilot study. Int J Sci Stud. 2018;5:34-8.
- [Google Scholar]
- ClinicalTrials.gov. 2016. Cyclosporine and Etanercept in Stevens-Johnson Syndrome and Toxic Epidermal Necrolysis (NATIENS) NCT02987257;. Available from: https://clinicaltrials.gov/ct2/show/study/NCT02987257 [Last accessed on 2019 May 12]
- [Google Scholar]
- Intravenous immunoglobulin in the treatment of Stevens-Johnson syndrome and toxic epidermal necrolysis: A meta-analysis with meta-regression of observational studies. Int J Dermatol. 2015;54:108-15.
- [CrossRef] [PubMed] [Google Scholar]
- Stevens-Johnson syndrome/toxic epidermal necrolysis: A multicenter retrospective study of 377 adult patients from the United States. J Invest Dermatol. 2018;138:2315-21.
- [CrossRef] [PubMed] [Google Scholar]






