Translate this page into:
Cyclosporine in Stevens Johnson syndrome and toxic epidermal necrolysis and retrospective comparison with systemic corticosteroid
2 Department of Dermatology, Armed Forces Medical College, Pune, Maharashtra, India
Correspondence Address:
G K Singh
Department of Dermatology, Venereology and Leprosy, Command Hospital, Eastern Command, Kolkata - 700 027, West Bengal
India
| How to cite this article: Singh G K, Chatterjee M, Verma R. Cyclosporine in Stevens Johnson syndrome and toxic epidermal necrolysis and retrospective comparison with systemic corticosteroid. Indian J Dermatol Venereol Leprol 2013;79:686-692 |
Abstract
Background: Stevens Johnson syndrome (SJS) and toxic epidermal necrolysis (TEN) are life-threatening dermatological emergencies. Many immunosuppressive modalities have been tried with variable results. Aims: To determine the efficacy of cyclosporine in cases of SJS and TEN and compare the efficacy with systemic corticosteroid in the same condition. Methods: Study was conducted at a tertiary hospital during 01 July 2011 to 30 June 2012. SCORTEN was assessed at the time of admission. Total body surface area (TBSA) assessment was like any burn patients. Cyclosporine was administered in the dose of 3 mg/kg body weight in three divided dosage for 07 days and then tapered over another 07 days. Data were compared to a historical series of SJS/TEN patients, managed by systemic steroids a year ago. Results: A total of 11 consecutive patients with a mean age of 32.09 and standard deviation (SD 16.17) were enrolled in to cyclosporine group, which were retrospectively compared to 6 patients with a mean age of 27.87 (SD 13.97) years in the corticosteroid group. The mean duration of re-epithelialization was 14.54 (SD 4.08) and 23 days (SD 6.68) in cyclosporine and corticosteroid group respectively (P = 0.009956). Mean hospital stay was 18.09 (SD 5.02) and 26 (SD 6.48) days in cyclosporine and corticosteroid group respectively (P = 0.02597). A total of 1.11 and 0.51 patients were expected to die against no death and two deaths in cyclosporine and corticosteroid group respectively (Standardized mortality ratio = 3.92) (P = 0.04321). Conclusion: This study definitely suggests that cyclosporine has encouraging role in the management of uncomplicated cases of SJS, SJS-TEN overlap or TEN.Introduction
Stevens Johnson syndrome (SJS) and toxic epidermal necrolysis (TEN) are life-threatening dermatological emergencies mainly due to drugs characterized by peeling of skin along with hemorrhagic crusting of lips and erosions of oral and genital mucosa. [1] World-wide, the average annual incidence of TEN is 0.4-1.3 cases per million populations. [2],[3] The mortality rate of SJS and TEN is high; approximately, 5% for SJS [4] and 30% for TEN. [5] Now SJS, SJS-TEN overlap and TEN are considered a spectrum of the same condition having common risk factors and causes, differentiated only by the extent of the body surface area (BSA) involved. Patients with epidermal detachment involving less than 10% of BSA are classified as having SJS, more than 30% BSA as TEN and 10-30% as SJS/TEN overlap. [6]
Apoptosis is believed to be the primary mechanism responsible for keratinocyte death in SJS/TEN. Two pathways have been proposed to support this theory. The first theory proposes that cytotoxic T-cells are activated by an inciting drug, which leads to the release of granzyme B and perforin, thereby activating the caspase cascade that ultimately results in keratinocyte apoptosis. [7] The second theory proposes that Fas-Fas ligand binding activates caspase 8, which results in nuclease activation and the widespread skin blistering characteristic of this severe drug reaction. [8]
A prognostic score called SCORTEN has been validated to demonstrate its ability to specifically predict patient outcome in SJS and TEN. [9],[10] Even though, some uncertainty still persists on effector mechanisms of TEN, the resemblance to graft rejection provided a rational for using the immunomodulating agents. [11] There are several studies illustrating variable results in the management of SJS/TEN. These included corticosteroids, [12] plasmapheresis, [13],[14] cyclophosphamide, [15] thalidomide. [16] Fas-Fas ligand and cytotoxic T-cell, which plays a vital role in the pathogenesis of SJS/TEN are respectively blocked by intravenous immunoglobulin (IVIG) and cyclosporine. Thus, theoretically making, IVIG and cyclosporine effective drugs in the management of SJS/TEN. [17] Several case reports have suggested encouraging results with IVIG in management of SJS/TEN. [18],[19],[20],[21],[22],[23],[24],[25],[26] However, study by Bachot et al. did not show any improvement with IVIG. [27] In Indian subcontinent managing SJS/TEN by IVIG is not cost-effective. In addition, there is no double-blind controlled trial, which suggest IVIG superior than other modalities. Several case reports and case series revealed encouraging result of use of cyclosporine in stopping disease progression and to prevent the mortality. [11],[28],[29],[30],[31],[32],[33],[34] In Indian subcontinent, systemic steroids have traditionally been used to manage this condition due to its experience of use, easy availability, and cost- effectiveness despite having multiple complications. This study was designed to evaluate the efficacy of cyclosporine and compare the results with patients who were managed by systemic steroids in tertiary health-care setting.
Methods
This was an open, pilot, and uncontrolled study. Study was conducted at a tertiary hospital of Kolkata during 01 July 2011 to 30 June 2012. Prior approval of ethical committee was taken. A total of 11 patients were enrolled into the study during this period. All cases fulfilling clinical diagnoses of SJS, SJS-TEN overlap, and TEN were included into the study. Exclusion criteria were prior treatment with any other immunosuppressive drugs, history of intolerance to cyclosporine, uncontrolled diabetes mellitus, Human immunodeficiency virus (HIV) positivity and cases of multi-organ failure and sepsis. It was decided in protocol that cyclosporine will be stopped if there is the development of high blood pressure with a diastolic pressure >110 mmHg and creatinine ≥150% of initial value. Irrespective of the clinical spectrum of disease (SJS/SJS-TEN overlap/TEN) cyclosporine was administered in solution form in the dose of 3 mg/kg body weight in three divided dosage for 07 days than 2 mg/kg body weight in two divided dosage for another 07 days. If there was no requirement of cyclosporine, it was to be stopped after 07 days of therapy. No other immunosuppressant was administered. Cases of SJS and SJS-TEN overlap were managed in the intensive care of Department of Dermatology while cases of TEN were managed in the burn center. It was proposed in the protocol that if there is clinical deterioration in the cases of SJS/SJS-TEN overlap, those would be managed in the burn center having intensive care facility. Barrier nursing, ambient temperature of 30°C, fluid and electrolyte balance and high calorie containing diets were considered in each patient. Injectable antibiotics were considered in strongly suspected or evident sepsis.
The patients were evaluated clinically daily for the entire period of hospitalization. Data were filled as per pre-designed proforma. Efficacy of cyclosporine was assessed by the average number of days in stabilization of disease progress, rate of re-epithelization of skin, duration of hospitalization, tolerance to treatment and rate of mortality at 1 month in comparison with the predicted death estimated by the SCORTEN at the time of admission. The actual death rates were compared to the predicted rates by standardized mortality ratio (SMR) analysis (sum of observed deaths/sum of expected deaths) × 100). The SCORTEN calculation was as per study of Bastuji-Garin et al. [10] Stabilization of disease was defined when new lesions cease to appear. Progression of disease was evaluated by any increase in erosions, blistering and positive Nikolsky′s sign. Re-epithelization was defined as complete healing of the skin without any erosion. Total body surface area (TBSA) assessment was like any burn patients, following rule of nine. Monitoring of patients was like well-established intensive care unit (ICU) protocol.
We compared the data with a historical series of the patients admitted to our hospital during the same period 1 year ago who were managed with systemic steroids in similar set up. The inclusion and exclusion criteria remained same as it was considered for the cyclosporine therapy except the fact these patients were managed by systemic steroid. These patients were treated with injectable dexamethasone followed by oral prednisolone in the dosage of ≥1 mg/kg/day. Epi-Info software 2007 was used for statistical analysis. Data were compared using the student′s two-tailed t-test and the P value less than 0.05 was considered significant.
Results
A total of 13 cases of SJS/TEN were seen during 01 July 2011 to 30 June 2012 who were treated by cyclosporine. 11 patients were included in to the study. 1 patient who did not fulfill the inclusion criteria was a case of multi-organ failure with sepsis that developed SJS-TEN overlap while being managed in the ICU and other patient was a case of HIV. No patient was dropped out from the study because of adverse effects of cyclosporine. All 11 patients survived and discharged from the hospital.
A total of 11 consecutive patients (six men and five women) were enrolled; they were aged 32.09 ± 16.17 years (mean±SD). Mean±SD delay between onset and admission was 2.63 ± 0.67 days (range 1-4). Only one case developed long-term complication that is corneal ulcer with symblepheron. There was no intolerance to cyclosporine. All five cases of SJS were given cyclosporine only for 07 days due to marked improvement in the clinical condition. Rest in other cases, full 14 days course, as proposed in the protocol was given.
There were total 9 cases of SJS, SJS-TEN overlap and TEN during 01 July 2010 and 30 June 2011 who were treated by corticosteroid. Out of which only six could be included into the study. In the excluded patients, first patient was administered more than one immunosuppressant, second was HIV positive and third one was a case of multi-organ failure being managed in the ICU. So, six patients (three male and three female) with a mean age of 27.87 (SD 13.97) were considered for comparison. Mean delay between the onset of the disease and admission was 2.16 (SD 0.75) days. Two patients died under this treatment regimen; however, there was no long-term complication in patients who survived the episode. Clinical profile, SCORTEN and clinical outcome parameters including means and standard deviation of the patients managed by cyclosporine and corticosteroid are depicted in [Table - 1] and [Table - 2] respectively.


Based on the SCORTEN system, 1.11 patients were expected to die with mean predicted mortality rate of 10.16 % (SD 9.5), in patients treated by cyclosporine, but no deaths were observed. SMR could not be calculated, because there was no death in this group. While in patients treated by corticosteroid, 0.51 patients were expected to die with mean predicted mortality rate 8.55 % (SD 13.10), but 02 deaths were observed (SMR 3.92). The comparison of mortality rate along with SCORTEN is depicted in [Table - 3].

The age and initial TBSA, which might have interfered with the clinical outcome, were also analyzed. There were no significant differences (P > 0.05). The time from the onset of the disease to admission was also not significantly different (P > 0.05). However, cyclosporine had significantly reduced the time to the arrest of progression of SJS/TEN (P = 0.04282), the total re-epithelization time (P = 0.009956) and hospitalization stay (P = 0.02597) in comparison to corticosteroid. There was no mortality in patients treated by cyclosporine in comparison to two deaths in the corticosteroid group and the difference was statistically significant (P = 0.04321). Those, who survived the disease, both drugs were tolerated well by the patients. Only one patient treated by cyclosporine developed corneal ulceration with symblepheron, which was statistically insignificant (P > 0.05) [Figure - 1]a and b. Pre-treatment and successful post-treatment of SJS-TEN complex in both groups is depicted in [Figure - 2]a, b, [Figure - 3]a and b.
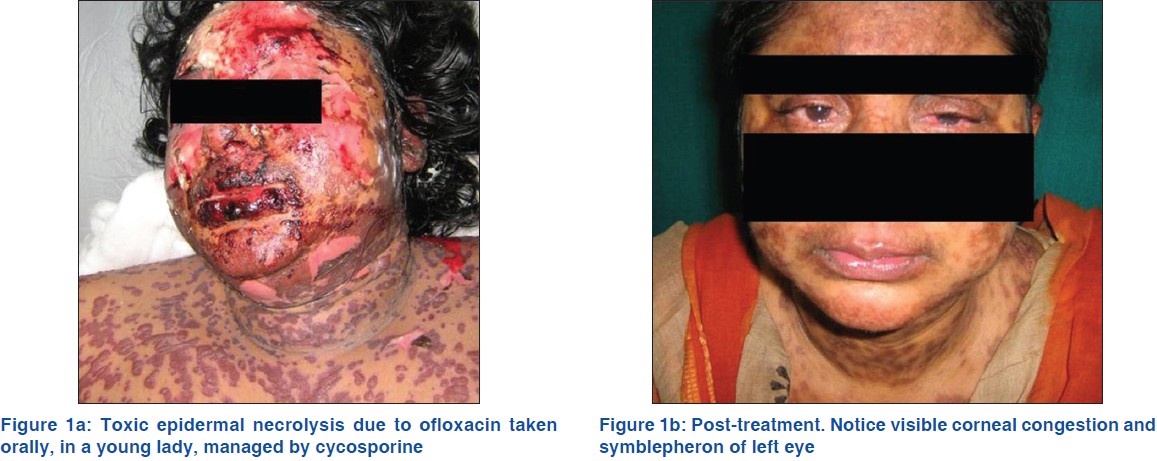 |
| Figure 1: |
 |
| Figure 2: |
 |
| Figure 3: |
Discussion
The Cochrane review on intervention for TEN revealed only one randomized controlled trial. [35] This trial compared the effectiveness of thalidomide with placebo. The only trial available used thalidomide, but this trial did not show any benefit from treatment compared against placebo, but highlighted increased chances of dying from the treatment. [16] Role of steroids in the management of TEN has been controversial. Several studies had shown possible benefit of corticosteroids. [12],[36],[37] However, off late most of the studies criticized the use of corticosteroids stating it not only prolongs the hospital stay, but also make patients susceptible for complications. [38],[39] A retrospective analysis of 289 patients from the EuroSCAR study found no benefit from corticosteroids or IVIG compared to supportive care alone. [40] Even, the combination therapy of IVIG and corticosteroid did not find any significant decrease in the mortality rate. [18]
In the paucity of data on effective drug for SJS/TEN prompt withdrawal of causative drugs should be a priority when managing such cases. Garcia-Doval et al. have shown that the earlier the causative drug is withdrawn, the better the prognosis, and that patients exposed to causative drugs with long half-lives have an increased risk of dying. [41] In order to identify the culprit drug(s), it is important to consider the chronology of administration of the drug and the reported ability of the drug to induce SJS/TEN. The reported ability or likelihood of a drug being the cause of SJS/TEN can be found in PubMed/MedLine or other appropriate sources such as the Litt′s drug eruption reference manual. [42] SJS/TEN is a life-threatening condition and therefore supportive care should be an essential part of the management strategy. [1]
Our study was distinct in the way, it had evaluated the efficacy of cyclosporine and compared historically to corticosteroids. It highlighted few important results. Cyclosporine was well tolerated by all the patients. There was no death in the patients managed by cyclosporine while there were two deaths in the corticosteroid group. All these results were statistically significant with P value less than 0.05. The only complication noted was a corneal ulceration and symblepheron formation. This was the lady who inadvertently continued to use ofloxacin eye drop which was culprit oral drug for the development of TEN. The same reason could explain her progression of BSA involvement in spite of being administered cyclosporine. 100 % survival in cyclosporine group could be explained by probable mechanism of action of this drug, which targets cytotoxic T-cell, which plays an important role in the apoptosis of keratinocytes. Other probable explanation could be better patient selection by excluding patients of multi-organ failure, sepsis, and HIV, which are the groups who succumb to death very fast when they develop SJS/TEN.
Recently, Valeyrie-Allanore et al. conducted an open, phase II trial to determine the safety and possible benefit of cyclosporine. [11] A total of 29 patients were included in the trial (10 SJS, 12 SJS-TEN overlap and 7 TEN), and 26 completed the treatment with cyclosporine administered orally (3 mg/kg/d for 10 days) and tapered over a month. The prognostic score predicted 2.75 deaths and none occurred (P = 0.1). There was no comparison with any historical group of corticosteroid. This study suggested that both the death rate and the progression of detachment seemed lower than expected, suggesting a possible usefulness of cyclosporine in SJS and TEN.
In a case series reported by Arévalo et al. [31] in which 11 patients treated enterally with cyclosporine 3 mg/kg daily observed a rapid epithelialization with no significant toxicity in comparison with patients treated with cyclophosphamide and corticosteroids combined (n = 6). Similar findings were noted by Reese et al. in four patients with SJS/TEN who were managed by cyclosporine. [43]
This study provided an excellent result with cyclosporine; however, comment on its efficacy cannot be made due to inherent constrain of the study design. An open, uncontrolled study with very small sample size in each group and selection of uncomplicated cases are obvious limitations of this study, which may have favored the better outcome of cyclosporine. A large, double-blind, placebo-controlled, randomized trial would be more appropriate to confirm its efficacy, which is not only unpractical, but also unethical. Like most of the recent studies our study also find use of corticosteroid in the management of SJS/TEN cause prolong hospital stay and increase in the mortality rate. This study definitely suggests that cyclosporine has encouraging role in the management of uncomplicated cases of SJS, SJS-TEN overlap or TEN.
| 1. |
Roujeau JC, Stern RS. Severe adverse cutaneous reactions to drugs. N Engl J Med 1994;331:1272-85.
[Google Scholar]
|
| 2. |
Abood GJ, Nickoloff BJ, Gamelli RL. Treatment strategies in toxic epidermal necrolysis syndrome: Where are we at? J Burn Care Res 2008;29:269-76.
[Google Scholar]
|
| 3. |
Mockenhaupt M, Viboud C, Dunant A, Naldi L, Halevy S, Bouwes Bavinck JN, et al. Stevens-Johnson syndrome and toxic epidermal necrolysis: Assessment of medication risks with emphasis on recently marketed drugs. The EuroSCAR-study. J Invest Dermatol 2008;128:35-44.
[Google Scholar]
|
| 4. |
Revuz J, Penso D, Roujeau JC, Guillaume JC, Payne CR, Wechsler J, et al. Toxic epidermal necrolysis. Clinical findings and prognosis factors in 87 patients. Arch Dermatol 1987;123:1160-5.
[Google Scholar]
|
| 5. |
Schöpf E, Stühmer A, Rzany B, Victor N, Zentgraf R, Kapp JF. Toxic epidermal necrolysis and Stevens-Johnson syndrome. An epidemiologic study from West Germany. Arch Dermatol 1991;127:839-42.
[Google Scholar]
|
| 6. |
Auquier-Dunant A, Mockenhaupt M, Naldi L, Correia O, Schröder W, Roujeau JC, et al. Correlations between clinical patterns and causes of erythema multiforme majus, Stevens-Johnson syndrome, and toxic epidermal necrolysis: Results of an international prospective study. Arch Dermatol 2002;138:1019-24.
[Google Scholar]
|
| 7. |
Pereira FA, Mudgil AV, Rosmarin DM. Toxic epidermal necrolysis. J Am Acad Dermatol 2007;56:181-200.
[Google Scholar]
|
| 8. |
Wehrli P, Viard I, Bullani R, Tschopp J, French LE. Death receptors in cutaneous biology and disease. J Invest Dermatol 2000;115:141-8.
[Google Scholar]
|
| 9. |
Guégan S, Bastuji-Garin S, Poszepczynska-Guigné E, Roujeau JC, Revuz J. Performance of the SCORTEN during the first five days of hospitalization to predict the prognosis of epidermal necrolysis. J Invest Dermatol 2006;126:272-6.
[Google Scholar]
|
| 10. |
Bastuji-Garin S, Fouchard N, Bertocchi M, Roujeau JC, Revuz J, Wolkenstein P. SCORTEN: A severity-of-illness score for toxic epidermal necrolysis. J Invest Dermatol 2000;115:149-53.
[Google Scholar]
|
| 11. |
Valeyrie-Allanore L, Wolkenstein P, Brochard L, Ortonne N, Maître B, Revuz J, et al. Open trial of ciclosporin treatment for Stevens-Johnson syndrome and toxic epidermal necrolysis. Br J Dermatol 2010;163:847-53.
[Google Scholar]
|
| 12. |
Patterson R, Miller M, Kaplan M, Doan T, Brown J, Detjen P, et al. Effectiveness of early therapy with corticosteroids in Stevens-Johnson syndrome: Experience with 41 cases and a hypothesis regarding pathogenesis. Ann Allergy 1994;73:27-34.
[Google Scholar]
|
| 13. |
Chaidemenos GC, Chrysomallis F, Sombolos K, Mourellou O, Ioannides D, Papakonstantinou M. Plasmapheresis in toxic epidermal necrolysis. Int J Dermatol 1997;36:218-21.
[Google Scholar]
|
| 14. |
Egan CA, Grant WJ, Morris SE, Saffle JR, Zone JJ. Plasmapheresis as an adjunct treatment in toxic epidermal necrolysis. J Am Acad Dermatol 1999;40:458-61.
[Google Scholar]
|
| 15. |
Heng MC, Allen SG. Efficacy of cyclophosphamide in toxic epidermal necrolysis. Clinical and pathophysiologic aspects. J Am Acad Dermatol 1991;25:778-86.
[Google Scholar]
|
| 16. |
Wolkenstein P, Latarjet J, Roujeau JC, Duguet C, Boudeau S, Vaillant L, et al. Randomised comparison of thalidomide versus placebo in toxic epidermal necrolysis. Lancet 1998;352:1586-9.
[Google Scholar]
|
| 17. |
Fernando SL. The management of toxic epidermal necrolysis. Australas J Dermatol 2012;53:165-71.
[Google Scholar]
|
| 18. |
Yang Y, Xu J, Li F, Zhu X. Combination therapy of intravenous immunoglobulin and corticosteroid in the treatment of toxic epidermal necrolysis and Stevens-Johnson syndrome: A retrospective comparative study in China. Int J Dermatol 2009;48:1122-8.
[Google Scholar]
|
| 19. |
Stella M, Clemente A, Bollero D, Risso D, Dalmasso P. Toxic epidermal necrolysis (TEN) and Stevens-Johnson syndrome (SJS): Experience with high-dose intravenous immunoglobulins and topical conservative approach. A retrospective analysis. Burns 2007;33:452-9.
[Google Scholar]
|
| 20. |
Tristani-Firouzi P, Petersen MJ, Saffle JR, Morris SE, Zone JJ. Treatment of toxic epidermal necrolysis with intravenous immunoglobulin in children. J Am Acad Dermatol 2002;47:548-52.
[Google Scholar]
|
| 21. |
Campione E, Marulli GC, Carrozzo AM, Chimenti MS, Costanzo A, Bianchi L. High-dose intravenous immunoglobulin for severe drug reactions: Efficacy in toxic epidermal necrolysis. Acta Derm Venereol 2003;83:430-2.
[Google Scholar]
|
| 22. |
Metry DW, Jung P, Levy ML. Use of intravenous immunoglobulin in children with Stevens-Johnson syndrome and toxic epidermal necrolysis: Seven cases and review of the literature. Pediatrics 2003;112:1430-6.
[Google Scholar]
|
| 23. |
Prins C, Kerdel FA, Padilla RS, Hunziker T, Chimenti S, Viard I, et al. Treatment of toxic epidermal necrolysis with high-dose intravenous immunoglobulins: Multicenter retrospective analysis of 48 consecutive cases. Arch Dermatol 2003;139:26-32.
[Google Scholar]
|
| 24. |
Al-Mutairi N, Arun J, Osama NE, Amr Z, Mazen AS, Ibtesam el-A, et al. Prospective, noncomparative open study from Kuwait of the role of intravenous immunoglobulin in the treatment of toxic epidermal necrolysis. Int J Dermatol 2004;43:847-51.
[Google Scholar]
|
| 25. |
Tan AW, Thong BY, Yip LW, Chng HH, Ng SK. High-dose intravenous immunoglobulins in the treatment of toxic epidermal necrolysis: An Asian series. J Dermatol 2005;32:1-6.
[Google Scholar]
|
| 26. |
Mangla K, Rastogi S, Goyal P, Solanki RB, Rawal RC. Efficacy of low dose intravenous immunoglobulins in children with toxic epidermal necrolysis: An open uncontrolled study. Indian J Dermatol Venereol Leprol 2005;71:398-400.
[Google Scholar]
|
| 27. |
Bachot N, Revuz J, Roujeau JC. Intravenous immunoglobulin treatment for Stevens-Johnson syndrome and toxic epidermal necrolysis: A prospective noncomparative study showing no benefit on mortality or progression. Arch Dermatol 2003;139:33-6.
[Google Scholar]
|
| 28. |
Zaki I, Patel S, Reed R, Dalziel KL. Toxic epidermal necrolysis associated with severe hypocalcaemia, and treated with cyclosporin. Br J Dermatol 1995;133:337-8.
[Google Scholar]
|
| 29. |
Sullivan JR, Watson A. Lamotrigine-induced toxic epidermal necrolysis treated with intravenous cyclosporin: A discussion of pathogenesis and immunosuppressive management. Australas J Dermatol 1996;37:208-12.
[Google Scholar]
|
| 30. |
Jarrett P, Rademaker M, Havill J, Pullon H. Toxic epidermal necrolysis treated with cyclosporin and granulocyte colony stimulating factor. Clin Exp Dermatol 1997;22:146-7.
[Google Scholar]
|
| 31. |
Arévalo JM, Lorente JA, González-Herrada C, Jiménez-Reyes J. Treatment of toxic epidermal necrolysis with cyclosporin A. J Trauma 2000;48:473-8.
[Google Scholar]
|
| 32. |
Robak E, Robak T, Góra-Tybor J, Chojnowski K, Strzelecka B, Waszczykowska E, et al. Toxic epidermal necrolysis in a patient with severe aplastic anemia treated with cyclosporin A and G-CSF. J Med 2001;32:31-9.
[Google Scholar]
|
| 33. |
Hashim N, Bandara D, Tan E, Ilchyshyn A. Early cyclosporine treatment of incipient toxic epidermal necrolysis induced by concomitant use of lamotrigine and sodium valproate. Acta Derm Venereol 2004;84:90-1.
[Google Scholar]
|
| 34. |
Rai R, Srinivas CR. Suprapharmacologic doses of intravenous dexamethasone followed by cyclosporine in the treatment of toxic epidermal necrolysis. Indian J Dermatol Venereol Leprol 2008;74:263-5.
[Google Scholar]
|
| 35. |
Majumdar S, Mockenhaupt M, Roujeau J, Townshend A. Interventions for toxic epidermal necrolysis. Cochrane Database Syst Rev 2002;CD001435.
[Google Scholar]
|
| 36. |
Kakourou T, Klontza D, Soteropoulou F, Kattamis C. Corticosteroid treatment of erythema multiforme major (Stevens-Johnson syndrome) in children. Eur J Pediatr 1997;156:90-3.
[Google Scholar]
|
| 37. |
Tripathi A, Ditto AM, Grammer LC, Greenberger PA, McGrath KG, Zeiss CR, et al. Corticosteroid therapy in an additional 13 cases of Stevens-Johnson syndrome: A total series of 67 cases. Allergy Asthma Proc 2000;21:101-5.
[Google Scholar]
|
| 38. |
Chave TA, Mortimer NJ, Sladden MJ, Hall AP, Hutchinson PE. Toxic epidermal necrolysis: Current evidence, practical management and future directions. Br J Dermatol 2005;153:241-53.
[Google Scholar]
|
| 39. |
Gerdts B, Vloemans AF, Kreis RW. Toxic epidermal necrolysis: 15 years' experience in a Dutch burns centre. J Eur Acad Dermatol Venereol 2007;21:781-8.
[Google Scholar]
|
| 40. |
Schneck J, Fagot JP, Sekula P, Sassolas B, Roujeau JC, Mockenhaupt M. Effects of treatments on the mortality of Stevens-Johnson syndrome and toxic epidermal necrolysis: A retrospective study on patients included in the prospective EuroSCAR Study. J Am Acad Dermatol 2008;58:33-40.
[Google Scholar]
|
| 41. |
Garcia-Doval I, LeCleach L, Bocquet H, Otero XL, Roujeau JC. Toxic epidermal necrolysis and Stevens-Johnson syndrome: Does early withdrawal of causative drugs decrease the risk of death? Arch Dermatol 2000;136:323-7.
[Google Scholar]
|
| 42. |
Litt JZ. Litt's Drug Eruptions and Reference Manual. 16 th ed., London: Informa Healthcare; 2010.
th ed., London: Informa Healthcare; 2010.'>[Google Scholar]
|
| 43. |
Reese D, Henning JS, Rockers K, Ladd D, Gilson R. Cyclosporine for SJS/TEN: A case series and review of the literature. Cutis 2011;87:24-9.
[Google Scholar]
|
Fulltext Views
9,805
PDF downloads
2,664





