Deciphering the landscape of hereditary angioedema in India: Perspective for Indian dermatologists
Corresponding author: Dr. Ankur Kumar Jindal, Department of Paediatrics, Advanced Paediatrics Centre, Postgraduate Institute of Medical Education and Research, Chandigarh, India. ankurjindal11@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Barman P, Gayathri CV, Sarkar R, Shenoy M, Jindal AK, Dogra S. Deciphering the landscape of hereditary angioedema in India: Perspective for Indian dermatologists. Indian J Dermatol Venereol Leprol. 2024;90:789-93. doi: 10.25259/IJDVL_1002_2023
Introduction
Hereditary angioedema (HAE) is an uncommon genetic disorder caused by deficiency/ dysfunction of C1-esterase inhibitor (C1-INH) protein.1,2 Most patients often seek their first consultation from dermatology.3,4 However, because of lack of awareness, most patients remain undiagnosed/ misdiagnosed.5 This paper provides an update on HAE from a dermatologist’s perspective and the availability of first-line treatment options in India.
How common is HAE in India?
Considering the global prevalence of HAE (1/10,000 to 1/150,000 populations) and the population of India (1.4 billion), the estimated number of patients with HAE range from 27,000 to 135,000.6-8 First case of HAE from India was reported in 1992 and ∼500 patients have been diagnosed till date.9
At our centre, we have been following up a cohort of ∼240 patients with HAE (approximately 25% patients diagnosed in childhood, <18 years old). Clinical profile of patients with HAE is comparable with published literature.3–5 However, our observations suggest that the abdominal symptoms are less commonly observed in the Indian setting (Indian Data is unpublished in literature).
What is the aetiopathogenesis of HAE?
SERPING1 gene encodes for the C1-INH protein and pathogenic variants in this gene may either lead to reduced formation (Type1 HAE) or production of dysfunctional C1-INH protein (Type2 HAE)2 leading to overproduction of bradykinin (vasoactive peptide) which in turn leads to formation of angioedema [Figure 1].2 HAE with normal-C1-INH protein and function is extremely rare and no case has been reported in India yet.10
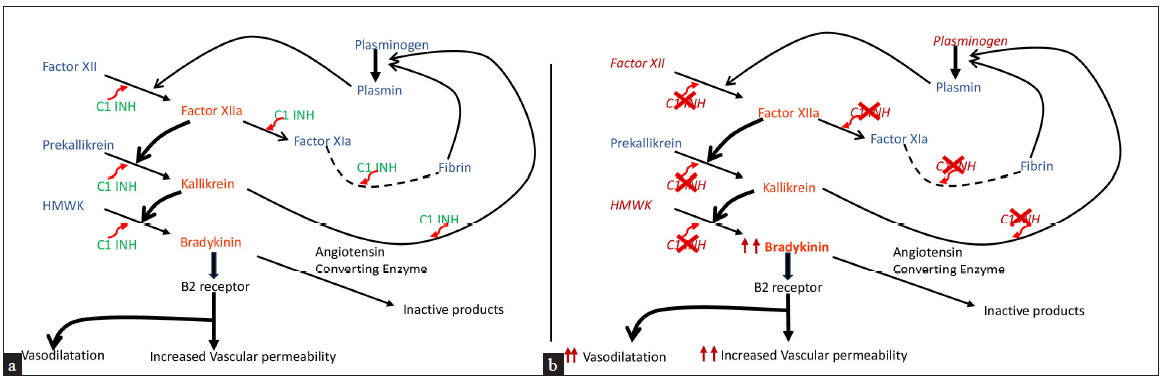
- (a) Normal regulation of the contact activation system and kallikrein/kinin system – inhibition of bradykinin levels by C1-esterase inhibitor at various levels; (b) increased bradykinin levels in hereditary angioedema due to impaired regulation of the contact activation system and kallikrein–kinin system.
What are the clinical manifestations of HAE?
Most patients (>50%) present in childhood or adolescence.3 In our previous publication, the median age of symptom onset and diagnosis was 6.25 years and 12 years, respectively.11 However, our recent experience suggests a slightly higher age of onset of symptoms and diagnosis. The delays in diagnosis continue to remain high (median delay >10 years) [unpublished data].
In contrast to the mast cell mediated angioedema [Figures 2a–2c], patients with HAE present with recurrent episodes of non-itchy asymmetric swellings involving extremities, face and genitalia, generally [Figure 2d-2f].12 Laryngeal oedema is a potentially life-threatening manifestation and may be the first presenting symptom.13
![(a), (b) and (c) depict allergic/mast cell–mediated angioedema involving [a] ear lobe, [b] urticarial rash over the abdominal wall and [c] peri-orbital region, respectively (d), (e) and (f) depict bradykinin-mediated angioedema due to hereditary angioedema involving peri-orbital region [(d), (e)], [d] lips and [f] left hand.](/content/126/2024/90/6/img/IJDVL-90-6-789-g2.png)
- (a), (b) and (c) depict allergic/mast cell–mediated angioedema involving [a] ear lobe, [b] urticarial rash over the abdominal wall and [c] peri-orbital region, respectively (d), (e) and (f) depict bradykinin-mediated angioedema due to hereditary angioedema involving peri-orbital region [(d), (e)], [d] lips and [f] left hand.
Abdominal episodes of HAE are often difficult to recognise3,14 and present with pain (often excruciating) and may be associated with vomiting, abdominal distension and diarrhoea.
The frequency of episodes of angioedema may increase during puberty (due to hormonal changes).3 Triggers such as emotional stress, trauma (pressure/physical contact during sports activity/ surgical procedures), viral infections, physical exertion, food (especially spices and high-protein diet) and drugs (such as oestrogens and angiotensin-converting enzyme inhibitors [ACEi]) have been reported.12
Prodromes are premonitory clinical manifestations that patients with HAE report a few hours before the onset of an attack. Erythema marginatum is an important prodromal symptom reported in >50% patients in Western literature.3,14
When to suspect HAE and where to refer?
HAE should be suspected if ≥1 of the following are present [Figures 2d, 2e, 2f]:
Recurrent episodes of non-itchy subcutaneous and/or submucosal swellings not associated with urticaria.
Family history of recurrent non-urticarial/non-itchy swellings.
Life-threatening laryngeal oedema.
Recurrent episodes of unexplained abdominal pain (associated with vomiting, diarrhoea, abdominal distension).
All patients with HAE need specialised care in a dedicated health care facility. There are two ‘Angioedema Centres of Reference and Excellence’ in India (Mumbai and Chandigarh). Under the auspices of the Hereditary Angioedema Society of India (https://haesi.in/) and HAE International (HAEi) organisation, a ‘Virtual Angioedema Centre’ has now been developed (https://ivac.haei.org).
What are the differential diagnoses for HAE?
The commonest cause of ‘angioedema’ in clinical practice is histamine/ mast cell–mediated angioedema [Figures 2a, 2b, 2c] [Table 1].3,12 Other differential diagnoses include acquired bradykinin-mediated angioedema (systemic lupus erythematosus and malignancies like lymphoma, drug-induced and idiopathic acquired angioedema.12 Patients with granulomatous cheilitis present with persistent lip swelling wherein diagnosis is established on lip biopsy.15 Occasionally, the swelling over hands and feet and abdominal symptoms in Henoch–Schonlein purpura may resemble HAE.16
| Characteristic | Mast cell mediator–mediated angioedema | Hereditary angioedema |
|---|---|---|
| Onset | Few seconds to a few minutes | Over several hours |
| Duration | Usually less than 24 hours | 2–6 days |
| Itching/urticaria | Always seen | Not seen |
| Laryngeal attack | Rare* | Common |
| Abdominal attacks | Rare | Common |
| Prodrome | Never seen | Distinct feature |
| Family history | Rare | Common |
How does one evaluate a patient suspected to have HAE?
International guidelines recommend estimation of C4 levels and quantitative and functional C1-INH assay in suspected patients with HAE.3 However, in resource-constrained settings, it may not be feasible to do all three tests in a given patient and a simplified algorithm is suggested [Figure 3].5,8 Estimation of C4 levels alone may be a cheap, simple and reliable screening test. However, C4 may be normal in approximately 20% patients with HAE.8 The sensitivity may increase if C4 levels are checked during an episode.
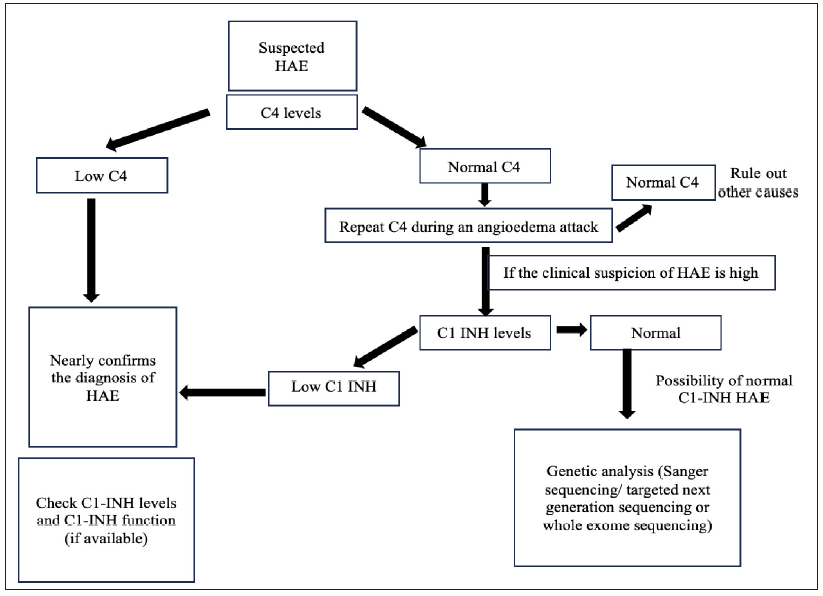
- Simplified algorithm for identifying patients with hereditary angioedema. (C1 INH- C1 esterase Inhibitor, HAE- Hereditary angioedema, C4- C4 complement protein.)
Genetic testing may be carried out depending on the availability of resources.3 Identification of genetic defects is essential for prenatal diagnosis and family screening and may be helpful for diagnosis in young children and during pregnancy.8
Screening of family members:
It is important to screen family members of a patient who has been diagnosed to have HAE regardless of symptoms.8 Sanger sequencing for a known variant in the SERPING1 gene may be a more cost-effective test for family screening as compared to C1-INH levels and C1-INH function.
What is the management strategy for a patient with HAE?
Management of HAE is usually divided into three broad categories:3
On-demand therapy (treatment of acute attack)
Short-term prophylaxis (STP) (prevention of attacks when they are anticipated)
Long-term prophylaxis (LTP) (prevention of attacks in patients with repeated angioedema attacks)
Are first-line treatment options for HAE available in India?
Intravenous plasma-derived (pd)-C1-INH is one of the first-line treatment option for patients with HAE.3,8 At present, three different preparations of pd-C1-INH are available in India. HAE has now been included in the list of diseases under the National Rare Disease Policy of the Government of India and pd-C1-INH can be accessed by all patients through the Centres of Excellence (COE) under this policy.
Pd-C1-INH needs to be stored either in a refrigerator (2–8℃) or at room temperature depending on the manufacturer’s instructions. After reconstitution, the injection has to be used as soon as possible preferably within 3 hours.
What should be done for a patient with HAE presenting with an acute attack?
International guidelines recommend treating all acute attacks of HAE.3 Intravenous pd-C1-INH is the drug of choice for on-demand treatment and should be administered as soon as possible, ideally within 1 hour of symptom onset and preferably by self-administration at home. Dose of pd-C1-INH is 10–20 IU/kg (adult dose 1000 IU). If there is inadequate response, a repeat dose can be given after 2–3 hours (max 60 IU/kg).8
The other recommended drugs are ecallantide (kallikrein inhibitor) [for patients ≥12 years of age] and icatibant (bradykinin B2 receptor antagonist) [for children and adults with HAE].3 These are not available in India as of now.
Can fresh-frozen plasma (FFP) be used for acute episodes of angioedema?
In countries where pd-C1-INH or other drugs are not available or accessible, FFP is still the best possible alternative for a life-threatening episode.8 The recommended dose is 20 mL/kg.3,8
Can we prevent the occurrence of angioedema in a patient with HAE?
(A) STP in HAE
STP must be available for all medical interventions including root canal treatment, endotracheal intubation, endoscopy or bronchoscopy. The drug of choice for STP is intravenous pd-C1-INH.3 There is minimal evidence to support the use of attenuated androgens (AA), tranexamic acid (TA) and FFP for STP. The intravenous pd-C1-INH dosage for children is 10–20 IU/kg (adult dose 1000 IU).
AA may be used to avoid an episode in a few high-risk scenarios, such as menstruation or travel, especially for patients who report specific triggers for their episodes. If a patient is already taking AA, the dose may be doubled, 2 days before the planned intervention or anticipated trigger and may be continued for 5 days. In patients who are not taking AA as LTP, 2 mg/day stanozolol or 10 mg/kg (maximum 600 mg) of danazol may be considered as STP.5
(B) LTP in HAE:
International guidelines recommend lanadelumab (monoclonal antibody against plasma kallikrein) [300 mg subcutaneously every 2–4 weeks] or oral berotralstat (plasma kallikrein inhibitor) [150 mg once daily] or intravenous pd-C1-INH as first-line therapy for LTP.3 However, lanadelumab and berotralstat are unavailable in India. AA, TA or a combination of the two would continue to be the cornerstone of LTP in India.8 Intravenous pd-C1-INH may be utilised when other drugs are contraindicated and the patient continues to suffer frequent episodes of angioedema.3 The suggested dose is 20 IU/kg twice weekly. The recommended doses for LTP with AA and TA are as follows:8
Stanozolol: 0.5 mg every other day up to 4 mg/day
Danazol: 100 mg every other day up to 600 mg/day
TA: 30–50 mg/kg/day in two to three divided doses with a maximum daily dose of 3 g/day
What are the mandatory precautions for patients with HAE?
ACEi and oestrogens must be avoided.8 Patients should be advised to identify triggers and avoid them as far as possible12. An ‘Emergency card’ that contains relevant information about clinical symptoms of HAE (especially laryngeal attack), emergency contact numbers and treatments (including on-demand therapy and requirement of tracheostomy/ cricothyrotomy) must be kept.17
Are there patient advocacy/support groups for HAE in India?
The first international patient advocacy group was formed in USA as HAEi (https://haei.org/) which has nearly 100 countries including India (HAE India, https://haeindia.haei.org/) as members. Their primary objectives are to raise awareness, improve diagnostics and ensure access of first-line medications to patients with HAE.1,8.
Conclusion
HAE must be suspected in a patient presenting with non-itchy/non-urticarial episodic swelling. Complement C4 is a simple and sensitive screening test for patients with HAE. First-line treatment options for HAE are now available in India and must be used to avoid mortality and to improve the quality of life. Efforts should be continued to bring better treatment options for HAE.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
References
- Hereditary angioedema: Diagnostic algorithm and current treatment concepts. Indian Dermatol Online J. 2021;12:796-804.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- An update on the genetics and pathogenesis of hereditary angioedema. Genes Dis. 2020;7:75-83.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- The international WAO/EAACI guideline for the management of hereditary angioedema—The 2021 revision and update. Allergy. 2022;77:1961-90.
- [CrossRef] [PubMed] [Google Scholar]
- Negative pressure flash pulmonary edema in a child with hereditary angioedema. Pediatr Allergy Immunol. 2022;33
- [CrossRef] [PubMed] [Google Scholar]
- Mitigating disparity in health-care resources between countries for management of hereditary angioedema. Clin Rev Allergy Immunol. 2021;61:84-97.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Hereditary angioedema: Epidemiology, management, and role of icatibant. Biol Targets Ther. 2013;7:103-13.
- [CrossRef] [PubMed] [Google Scholar]
- Epidemiology, management, and treatment access of hereditary angioedema in the asia pacific region: Outcomes from an international survey. J Allergy Clin Immunol Pract. 2023;11:1253-60.
- [CrossRef] [PubMed] [Google Scholar]
- Management of hereditary angioedema in resource-constrained settings: A consensus statement from Indian subcontinent. Asia Pac Allergy. 2023;13:60-5.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Hereditary angioedema in a family. J Assoc Physicians India. 1992;40:270-1.
- [PubMed] [Google Scholar]
- Novel hereditary angioedema linked with a heparan sulfate 3-O-sulfotransferase 6 gene mutation. J Allergy Clin Immunol. 2021;148:1041-8.
- [CrossRef] [PubMed] [Google Scholar]
- Novel SERPING1 gene mutations and clinical experience of type 1 hereditary angioedema from North India. Atanaskovic‐Markovic M, editor. Pediatr Allergy Immunol. 2021;32:599-611.
- [CrossRef] [PubMed] [Google Scholar]
- Differences and similarities in the mechanisms and clinical expression of bradykinin-mediated vs. mast cell–mediated angioedema. Clin Rev Allergy Immunol. 2021;61:40-9.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- clinical studies of sudden upper airway obstruction in patients with hereditary angioedema due to C1 esterase inhibitor deficiency. Arch Intern Med. 2003;163:1229.
- [CrossRef] [PubMed] [Google Scholar]
- The enigma of Prodromes in Hereditary Angioedema (HAE) Clin Rev Allergy Immunol. 2021;61:15-28.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Initial manifestations and short term follow-up results of Henoch-Schönlein purpura in children: A report from two centers. North Clin Istanb. 2020;7:341-7.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- WAO Guideline for the management of hereditary angioedema. World Allergy Organ J. 2012;5:182-99.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]





