Translate this page into:
Dermatoses with “collarette of skin”
Correspondence Address:
Arun C Inamadar
Department of Dermatology, Venereology and Leprosy, Shri B. M. Patil Medical College, Hospital and Research Center, BLDE University, Vijayapur - 586 103, Karnataka
India
| How to cite this article: Adya KA, Inamadar AC, Palit A. Dermatoses with “collarette of skin”. Indian J Dermatol Venereol Leprol 2019;85:116-124 |
A collarette in dermatology refers to “a narrow rim of loosened keratin overhanging the periphery of a circumscribed skin lesion, attached to the normal surrounding skin.”[1] The outer margin of the “collarette” is adherent while the inner margin is free. In practice, however, the skin forming the collarette may be scaly (e.g., pityriasis rosea, keratolysis exfoliativa, etc.) or thick and ridge-like, forming a rim around the lesion (e.g., acral fibrokeratoma, viral warts, etc.). While many disorders primarily exhibit such a morphology [Table - 1], it may be a secondary or a nonspecific manifestation in certain others.
Disorders of Keratinization
Pityriasis rosea
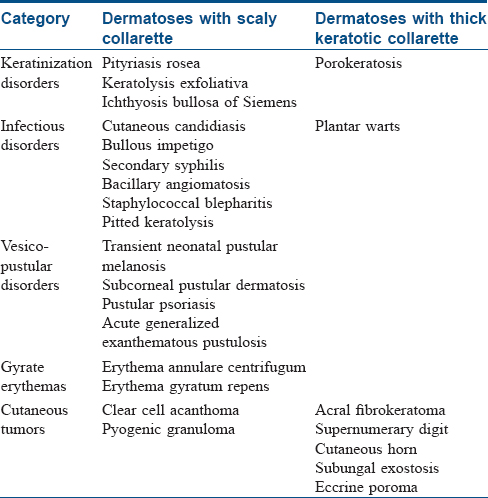
Pityriasis rosea is a common, acquired, self-limiting papulosquamous disorder commonly affecting children and young adults. A possible infective role has been speculated in its pathogenesis but has not been proven unequivocally. Classically, the disease begins as an erythematous macule with a peripheral rim of fine scaling that enlarges over the next few days into an annular to oval patch (“herald patch,” “mother patch”), commonly involving the trunk. The scaling appears as a collarette and characteristically lags behind the outer erythematous edge of the patch – “trailing scale” or the “hanging curtain sign” [Figure - 1]. In the next 1–2 weeks, smaller multiple pruritic lesions, similar to the herald patch, develop predominantly on the trunk extending up to the proximal arms. On the back, the long axes of these lesions are aligned parallel to the relaxed skin tension lines giving a “Christmas tree” appearance.[2],[3] The disease is self-limiting and resolves spontaneously in approximately 6 weeks.[2] Atypical forms have been described. Although innocuous, pityriasis rosea occurring in the first trimester of pregnancy may account for an increased risk of spontaneous abortion, preterm delivery and infantile hypotonia and hyporeactivity.[4],[5] A pityriasis rosea-like drug eruption can occur with certain medications as well.[6]
 |
| Figure 1: Pityriasis rosea |
Keratolysis exfoliativa
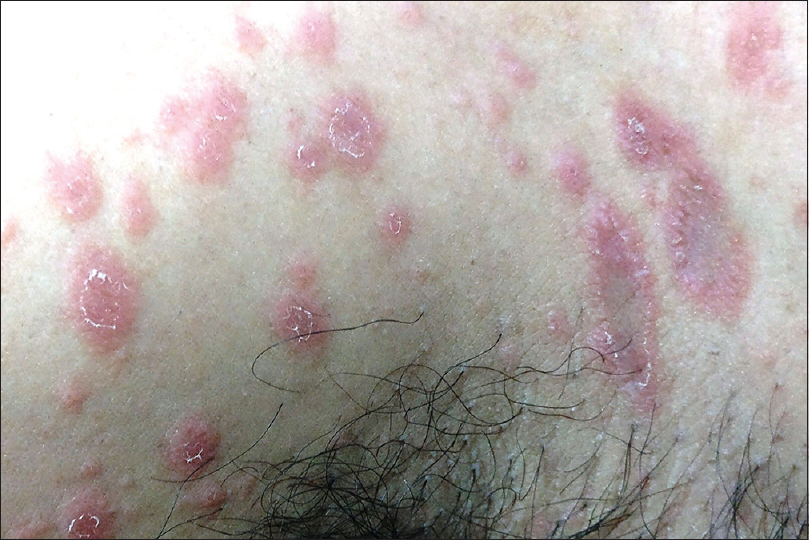
Keratolysis exfoliativa is a common, noninflammatory, focal or diffuse peeling of the skin of palms and less commonly of the soles. It is usually asymptomatic, commonly affecting healthy individuals. It is common in summer and worsened by warm climate. Clinically, it is characterized by discrete air-filled tiny vesicles that rupture and extend in an irregular annular or circinate pattern for up to 10 mm, with a characteristic peripheral collarette of scaling [Figure - 2]. Keratolysis exfoliativa is believed to be a milder variant of dyshidrotic eczema or is associated with atopy. Chang et al. suggest the disorder to be a distinct peeling disorder occurring mainly due to premature corneodesmolysis.[7],[8],[9]
 |
| Figure 2: Keratolysis exfoliativa |
Ichthyosis bullosa of Siemens (superficial epidermolytic ichthyosis)
Ichthyosis bullosa of Siemens is an autosomal dominant disorder of keratinization that is regarded as a superficial variant of bullous congenital ichthyosiform erythroderma (epidermolytic hyperkeratosis). It occurs due to mutation in the keratin 2e gene. Clinically, it is characterized by superficial trauma-induced blisters that spontaneously rupture leaving behind denuded skin with annular collarette-like scaling at the margins, classically referred to as mauserung phenomenon (epidermal moulting). The flexures, lower abdomen and the pretibial areas are commonly involved which may also exhibit a grayish, rippled, scaly hyperkeratosis. The disease usually begins in the neonatal period and extends into the childhood, and may occasionally persist until adulthood. Histopathologic features are similar to those in epidermolytic hyperkeratosis, but are confined to the superficial epidermis. Vacuolated keratinocytes in the upper spinous layer are an important diagnostic clue.[10],[11]
Porokeratosis
Porokeratosis is a group of keratinization disorders characterized by a common manifestation of marginate keratotic collarette that histopathologically corresponds to vertical columns of parakeratosis (coronoid lamellae). Different clinical forms of porokeratosis can be broadly grouped into disseminated (actinic, of childhood and of the immunosuppressed) and localized (of Mibelli, linear, punctate, giant and palmoplantar) forms. The disseminated actinic type is the commonest form of porokeratosis. Except for the punctate form, all the types exhibit a common pathognomonic rim of a keratotic ridge surrounding a central reddish, dry and atrophic skin [Figure - 3]. The peripheral ridge may be furrowed as well. This distinct feature of porokeratosis is conspicuously visualized by dermoscopy, which circumvents the need for a biopsy even in clinically atypical cases. Squamous cell carcinoma may rarely develop in long-standing lesions, with a greater frequency in the linear and giant forms.[12],[13],[14]
 |
| Figure 3: Porokeratosis |
Infectious Disorders
Cutaneous candidiasis
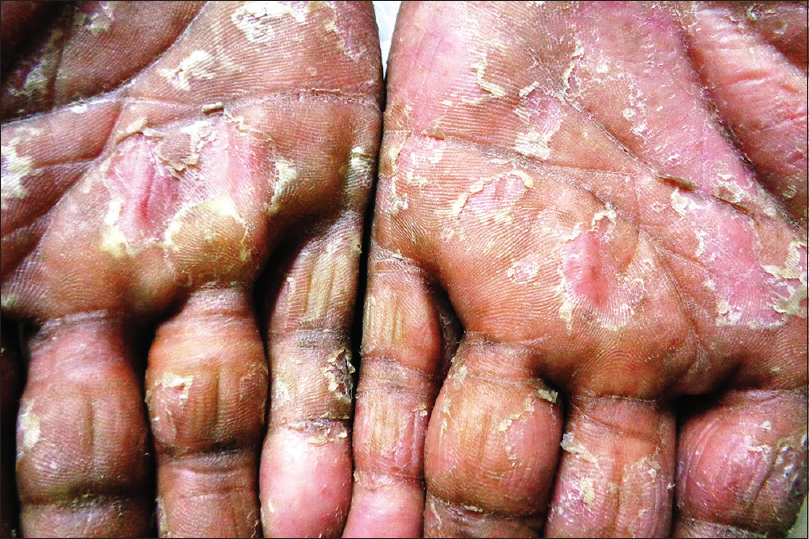
Candidiasis refers to an infection caused commonly by the yeast Candida albicans. Cutaneous candidiasis can manifest as congenital and neonatal candidiasis, diaper dermatitis and candidal intertrigo. Clinically, the lesions appear as erythematous eroded patches with marginal and satellite pustules. The pustules rupture to form a fringed irregular scalloped edge with a collarette of scaling [Figure - 4]. The anogenital region is commonly involved and lesions involving the crural folds invariably exhibit deep central clefting.[15],[16]
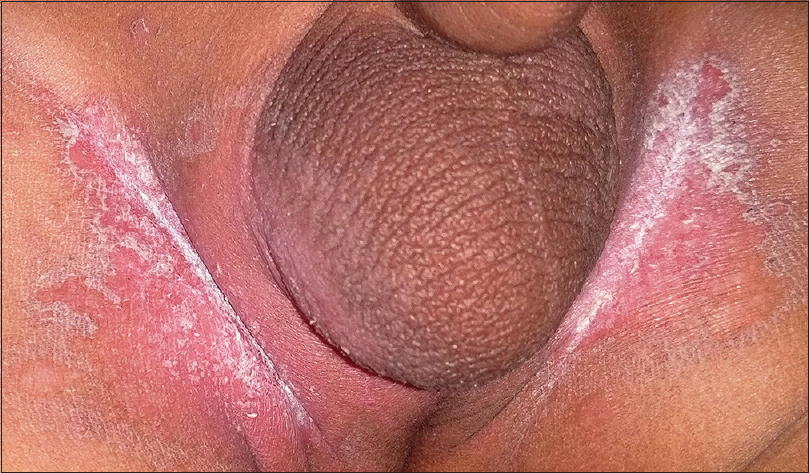 |
| Figure 4: Candidal intertrigo. Note the characteristic scalloped border with collarette of scales |
Bullous impetigo
Impetigo refers to a superficial bacterial infection, mainly occurring in two clinical forms: non-bullous and bullous. While the former is caused by both Staphylococci and Streptococci, the latter is caused by the epidermolytic toxin producing strains (group II, phage types 71, 55, 3A and 3C) of Staphylococcus aureus that hydrolyze desmoglein 1 leading to flaccid intraepidermal blistering. Bullous impetigo commonly affects children aged between 2 and 5 years. Although any site including the palms and soles may be affected, the flexures are frequently involved where the lesions begin as tiny vesicles enlarging to form flaccid bullae measuring up to 2 cm. Initially, the bullae contain clear fluid that later becomes purulent. Following the rupture of the bullae, raw erosions and crusting is seen with the reminder of the blister forming a pathognomonic collarette at the margin of the erosion [Figure - 5].[17],[18],[19]
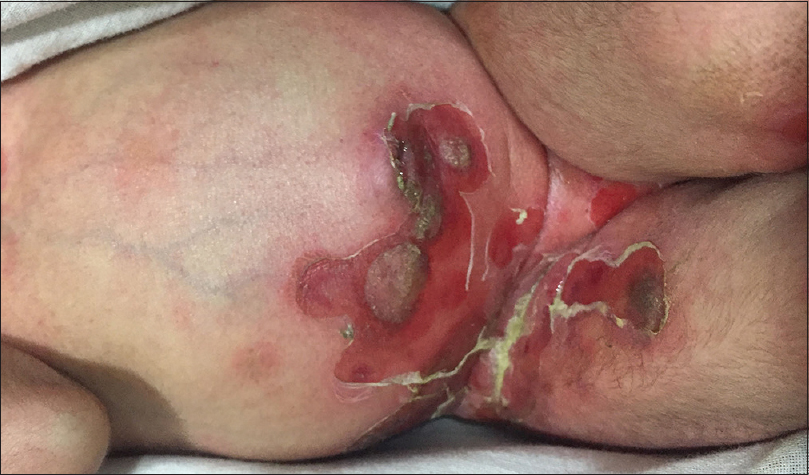 |
| Figure 5: Bullous impetigo |
Secondary syphilis
Syphilis is a sexually transmitted disease caused by the spirochete Treponema pallidum occurring in primary, secondary and tertiary stages, with a long and chronic course in the untreated. Papular lesions are the most prominent cutaneous manifestations of the secondary stage of syphilis that follow an evanescent coppery-red macular rash. The papular lesions involve the trunk and extremities, including the palms and soles. They are characterized by erythematous or brownish-red 0.5–2 cm discrete papules often demonstrating a pathognomonic collarette of scale on the surface [Figure - 6]a which is more conspicuous on the palmoplantar lesions. This collarette of scaling was first described by a Swiss-born French dermatologist Laurent-Théodore Biett and is named after him as “Biett's collarette” or “Biett's sign.” Dermoscopically, the collarette appears as a continuous white ring of scales encircling a central erythematous papule with a surrounding rim of erythema [Figure - 6]b.[20],[21],[22]
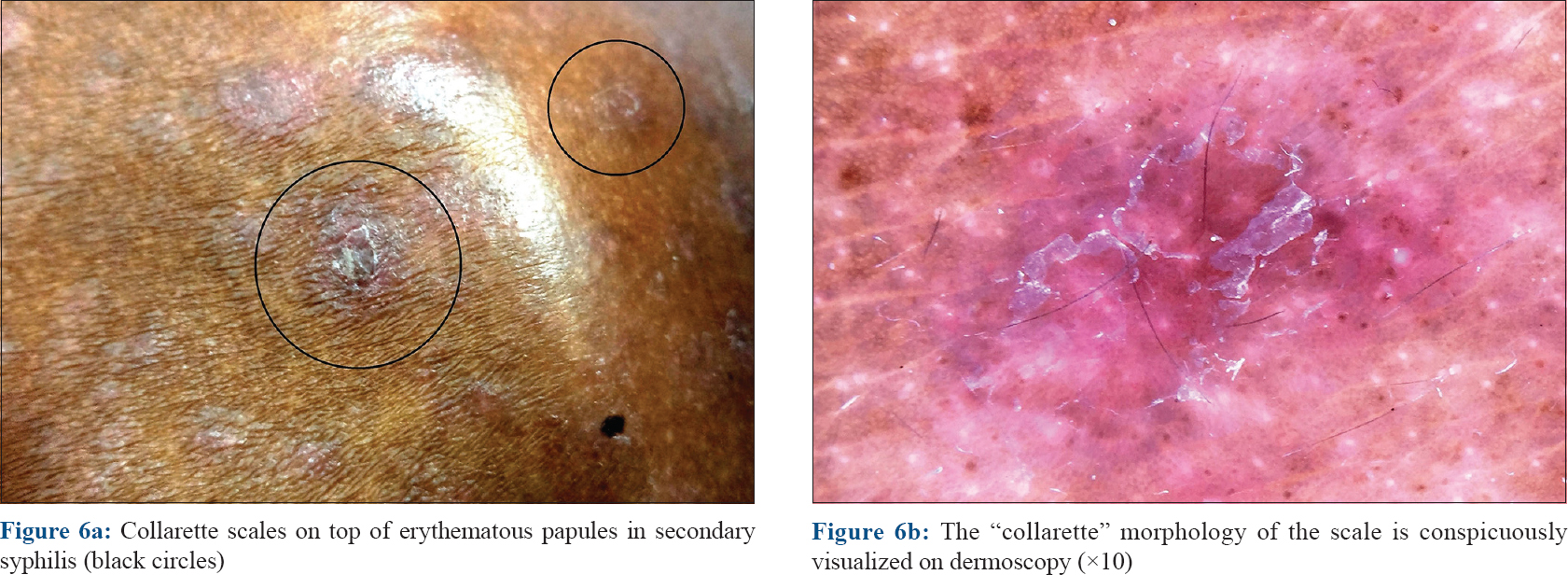 |
| Figure 6 |
Bacillary angiomatosis
Bacillary angiomatosis is primarily a vasoproliferative disease, seen in human immunodeficiency virus infected patients (with a CD4+ cell count of <50 mm−3) caused by Bartonella henselae and Bartonella quintana. Cutaneous bacillary angiomatosis manifest in various forms. Disseminated angiomatous papules resembling pyogenic granuloma are the commonest. They are tender, often pedunculated and surrounded by a collarette of scales.[23],[24],[25],[26] Similar lesions may involve the viscera as well.[27] Skin biopsy reveals aggregates of plump endothelial cells with luminal differentiation in a pale stroma. The pathognomonic feature is the presence of neutrophilic infiltrate throughout the lesion associated with smudgy amphophilic areas representing the organisms which stain with Giemsa, Warthin-Starry and Grocott's methenamine silver stains.[26],[28]
Staphylococcal blepharitis
Staphylococcal blepharitis is commonly caused by Staphylococcus aureus and Staphylococcus epidermidis. There is usually a unilateral localized erythema and edema of the anterior lid margin associated with the pathognomonic brittle fibrinous scales forming collarette around the base of the cilia. As the eyelashes grow, the adherent scales are lifted up. Other features that may be seen include dilated blood vessels on the lid margin arranged in rosettes, madarosis, localized ulceration and/or poliosis. The infection may extend to the posterior lid margin, and then onto the conjunctiva and periphery of the cornea, manifesting as follicular conjunctivitis and marginal keratitis.[29],[30],[31]
Pitted keratolysis
Pitted keratolysis is a superficial bacterial infection caused by a variety of organisms (Kytococcus sedentarius, Dermatophilus congolensis, Actinomyces and Corynebacterium spp.) that commonly affects the weight-bearing areas of soles in hot and humid climates. The lesions at these sites are seen as malodorous discrete superficial pits that may coalesce into larger noninflammatory erosions. Lesions can uncommonly occur on the palms and nonweight-bearing areas of the feet where they typically appear as collarette of scales rather than pits resembling keratolysis exfoliativa (see above).[32],[33],[34],[35]
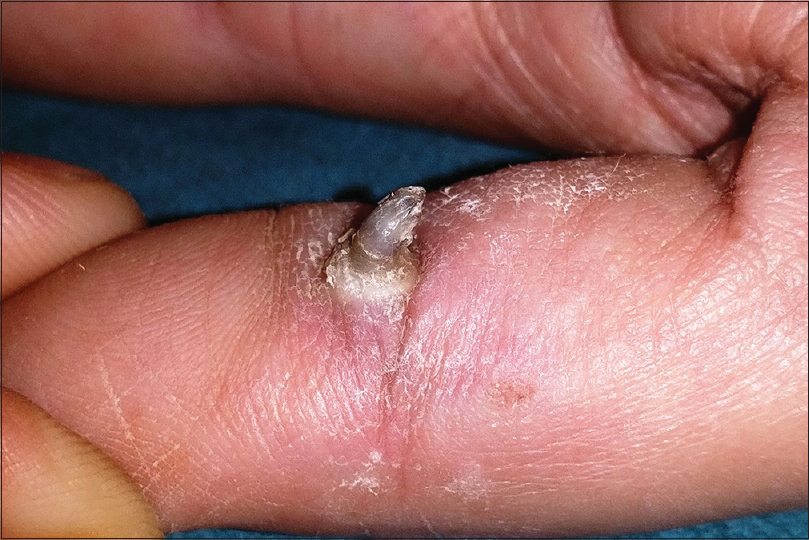
Plantar warts
Plantar warts are commonly caused by the human papilloma virus 1. They may be difficult to distinguish at times from corns and callosities. However, plantar warts obliterate the dermatoglyphics and demonstrate a soft core (as opposed to the hard core in corns) with pin-point bleeding spots or intracapillary thrombosis on superficial pairing of the lesion (not seen with corns and calluses). Plantar warts are sharply demarcated and exhibit a collarette of raised normal surrounding skin. Superficial pairing of the wart surface through this collarette shows abrupt ending of the epithelial ridges of the normal plantar skin at this point [Figure - 7]. A thick raised keratinous collarette may also be seen surrounding an old wart elsewhere on the body too.[4],[36],[37],[38],[39],[40],[41],[42],[43],[44],[45],[46],[47],[48]
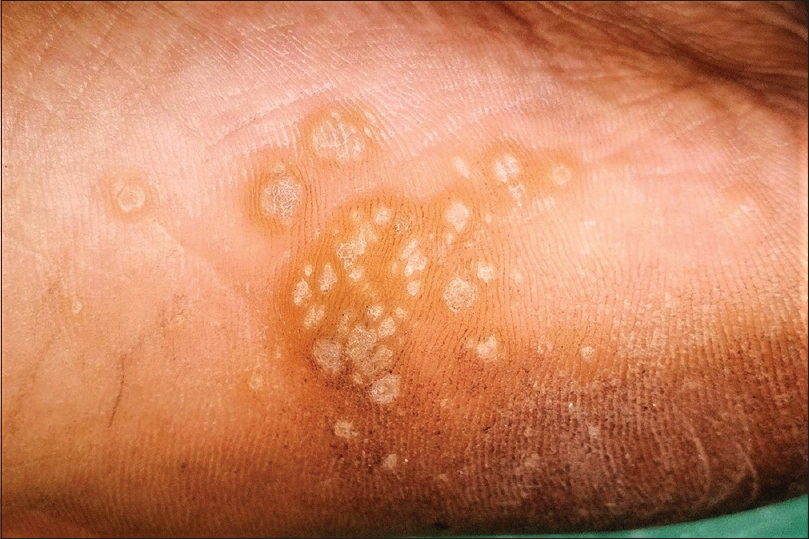 |
| Figure 7: Plantar warts with colarette of raised surrounding normal skin. Note the abrupt termination of dermatoglyphics at the inner margin of the collarette. |
Vesico-pustular Disorders
Transient neonatal pustular melanosis
Transient neonatal pustular melanosis is one of the transient neonatal pustuloses, characterized by superficial noninflammatory vesicles or pustules that rupture spontaneously leaving behind a characteristic collarette of white scales. These scales are later replaced by postinflammatory hyperpigmentation that resolves gradually over several weeks. The head and neck region and/or the lower extremities are commonly involved, and the condition is almost exclusive to full-term neonates of African descent. Some authors believe it to be a variant of erythema toxicum neonatorum; however, most of the lesions are seen at birth, and the sterile pustules are composed of neutrophils as opposed to the eosinophils in erythema toxicum neonatorum.[39],[40]
Subcorneal pustular dermatosis (Sneddon-Wilkinson disease)
Subcorneal pustular dermatosis is an uncommon, chronic, remitting and relapsing pustular dermatosis commonly affecting the middle-aged females. Clinically, the lesions appear as flaccid sterile pustules on either normal or erythematous skin. Lesions usually favor the flexures and appear in crops with a tendency to coalesce forming various patterns – annular, polycyclic, serpiginous or circinate. A typical feature is the formation of a fluid level within the blister as the pus accumulates in the dependent portion and the clear fluid on top. The lesions rupture in a few days and dry up to form crusts and collarette scaling. Histopathologically, there is accumulation of neutrophils just beneath the stratum corneum.[41],[42]
Pustular psoriasis
Pustular psoriasis, either generalized or localized, is characterized by development of sterile pustules on a patchy or confluent erythema. The pustules begin as discrete tiny lesions that may coalesce to form lakes of pus. In a few days, they rupture and dry up, leaving behind erosions surrounded by collarette of scaling [Figure - 8]. In the generalized forms (von Zumbusch and impetigo herpetiformis), the skin lesions are preceded by fever and systemic complaints. The pustules favor the trunk and extremities, and typically appear in waves accompanied by fever. The localized form of pustular psoriasis commonly involves the hands and feet (palmoplantar pustulosis and acrodermatitis continua of Hallopeau) and although not associated with any systemic effects, the chronic and persistent nature of the disease significantly affects the patients' quality of life [Figure - 9].[43],[44]
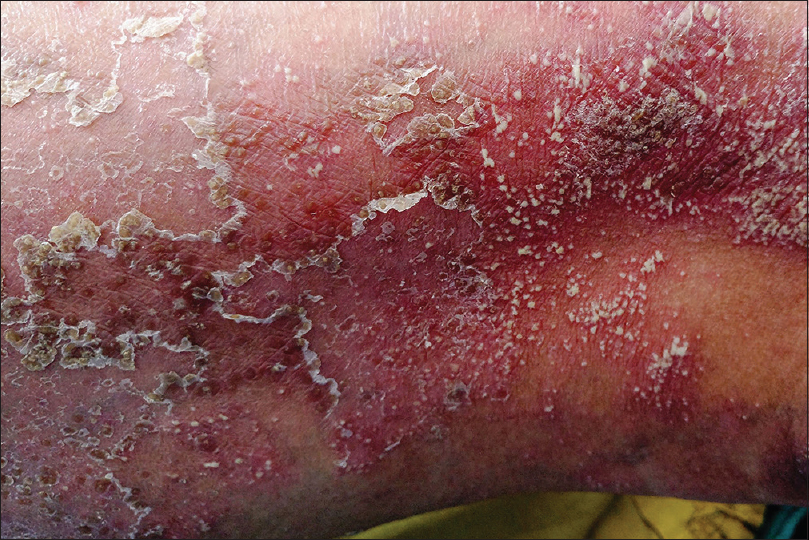 |
| Figure 8: Pustular psoriasis |
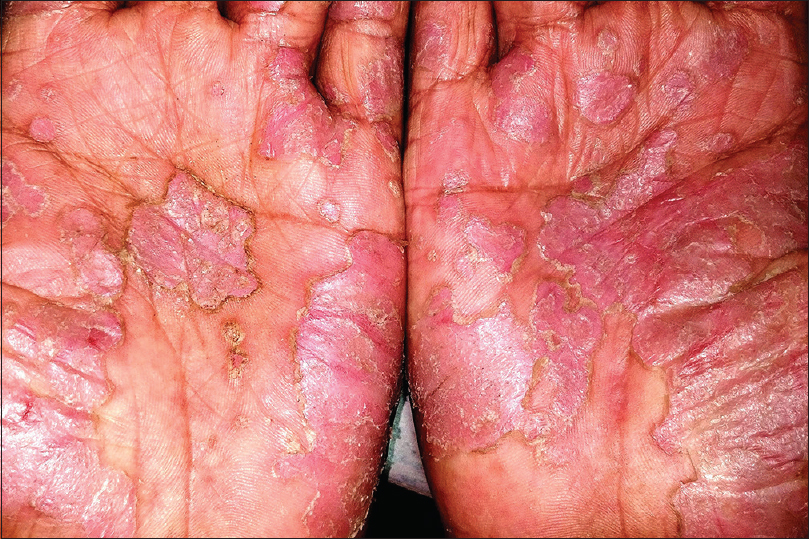 |
| Figure 9: Palmar pustulosis |
Acute generalized exanthematous pustulosis
Acute generalized exanthematous pustulosis is an acute febrile exanthematous pustulosis, commonly occurring as an adverse cutaneous drug reaction. Aminopenicillins, sulfonamides, quinolones, hydroxychloroquine and calcium channel blockers are the frequently implicated drugs. Lesions generally appear in 24–48 h following the intake of the offending drug, frequently in the flexures as tiny nonfollicular pustules on an erythematous base that gradually extend onto the trunk and extremities. The lesions involute spontaneously in a couple of weeks leaving behind the typical collarette of scaling. The disorder is usually a benign one, though secondary infection of the lesions can complicate the otherwise uneventful course.[45],[46],[47]
Gyrate Erythemas
Erythema annulare centrifugum
Erythema annulare centrifugum is a reactive migratory erythema that has been speculated to be associated with certain infections (Epstein-Barr virus infection, herpes zoster, molluscum contagiosum, candidiasis, dermatophyte infections, etc.), drugs (piroxicam, hydroxychloroquine sulfate, hydrochlorothiazide, amitriptyline, etc.) and certain other disorders such as sarcoidosis, hepatic and thyroid disorders, hematological and solid organ malignancies. However, none of these associations have been proven beyond doubt. It is divided into a superficial type (of Colcott Fox) and a deep type (of Darier) with distinct clinical and histopathological features. The disease commonly affects adults and the lesions favor the buttocks, thighs and trunk. The lesions begin as erythematous papules that slowly migrate and enlarge centrifugally reaching up to 10 cm in diameter with central clearing. The peripheral margin is raised enough to be just palpable or thick cord-like. The superficial variant exhibits the typical trailing collarette scales (as in pityriasis rosea and histologically demonstrates spongiosis, parakeratosis and superficial perivascular mononuclear infiltrate. The deep variant lacks the scaling, and histopathologically is typified by the dense superficial and deep perivascular infiltrate (giving a “coat-sleeve” appearance) without any epidermal changes.[48],[49],[50],[51]
Erythema gyratum repens
Erythema gyratum repens is a paraneoplastic inflammatory gyrate erythema occurring most commonly in association with carcinoma of lung. It is seen more commonly in males, beyond the fourth decade. It has also been reported with other hematological and solid organ malignancies. Clinically, the trunk, neck and extremities are commonly involved and the lesions are characterized by intensely pruritic migratory erythematous concentric wave-like bands. The characteristic feature is the rapid migratory rate of the leading edge (1 cm/day) and development of new gyrate lesions within preexisting ones, giving an appearance of concentric bands resembling wood grain. The outermost edge shows collarette scales which may be trailing the edge. Although histopathological features of erythema gyratum repens are imprecise, the distinctive clinical characteristics, however, warrant evaluation of the patient for an underlying neoplasia. Resection of the tumor results in resolution of the lesions.[52],[53],[54]
Cutaneous Tumors
Clear cell acanthoma (pale cell acnathoma, degos tumor)
Clear cell acanthoma was first described by Degos et al. in 1962.[55] The tumor commonly affects the legs and feet of middle-aged men and women. It usually appears as a solitary circumscribed dome-shaped papule or nodule with pigmented scaly surface and a characteristic collarette of white scales surrounding the lesion. Multiple, polypoid and giant forms have also been described. Dermoscopy of the tumor shows central blood vessels oriented perpendicular to the skin surface and a peripheral squamous collarette. Similar features may be seen in psoriasis and in Bowen's disease and hence, a clinical correlation and histopathological examination is necessary for definitive diagnosis. The distinctive histopathological features of Degos tumor include an acanthotic epidermis with abundant clear cells, spongiosis, hypo- or agranulosis and elongation of rete ridges. The clear cells are polyhedral and larger than the keratinocytes and show positivity and sensitivity to periodic-acid Schiff staining and diastase reaction, respectively.[56],[57]
Pyogenic granuloma
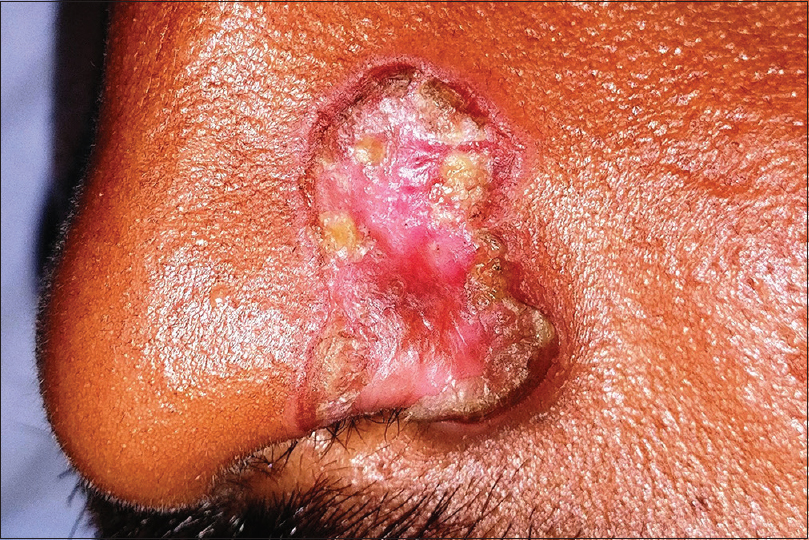
Pyogenic granuloma is a common acquired benign vascular tumor appearing mostly as rapidly growing, solitary, friable sessile or pedunculated vascular papules or nodules. A surrounding collarette of scale is seen at the base of the lesion in majority of the cases [Figure - 10]. Pyogenic granuloma is seen commonly in children and young adults with frequent involvement of the extremities and face. Trauma is the most frequent implicated factor in its etiopathogenesis. Lobular proliferation of capillary-like vessels in a loose stroma is the pathognomonic histopathological feature.[28],[58],[59] Dermoscopy shows central vascular area and a peripheral collarette. The central vascular area may show different patterns – red homogenous area, ulceration with hemorrhagic crusts or white “rail lines” on a reddish homogenous background intersecting the lesion.[60]
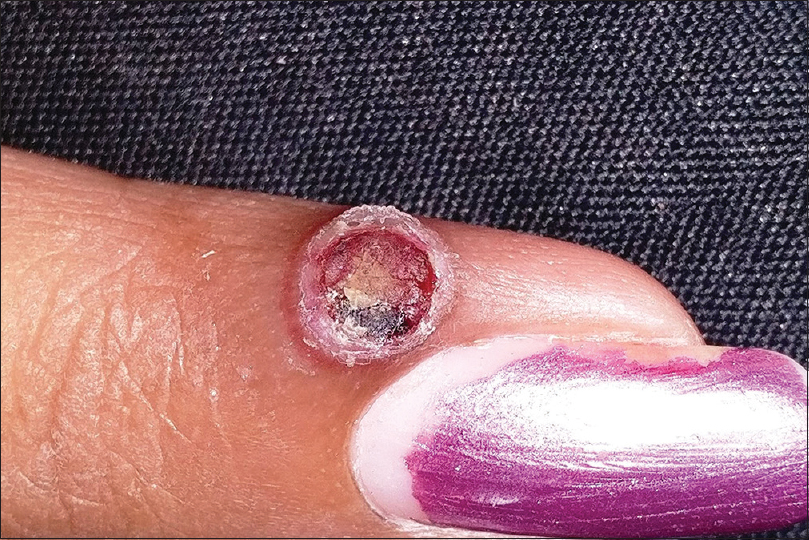 |
| Figure 10: Pyogenic granuloma |
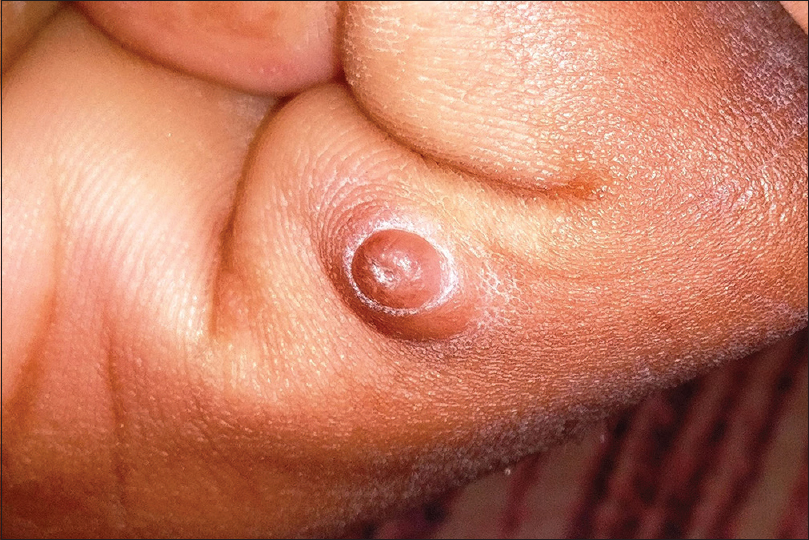
Acral fibrokeratoma
Acral fibrokeratoma (acquired digital fibrokeratoma) is a solitary benign tumor of adults frequently affecting the fingers and toes. The distinctive feature of the tumor is the moat-like collarette of thick raised skin surrounding a central dome-shaped papule or nodule with a warty surface [Figure - 11]. Dermoscopic features include a central homogenous pale yellow area with marginal white scaly collarette. Acral fibrokeratoma clinically resembles various other cutaneous tumors such as eccrine poroma, pyogenic granuloma [Figure - 10], a rudimentary supernumerary digit [Figure - 12], viral wart [Figure - 7], dermatofibroma and a cutaneous horn [Figure - 13]. Therefore, histopathological analysis is required for definitive diagnosis which shows a central area of increased vertically oriented collagen fibers with interspersed blood vessels underlying a hyperkeratotic and acanthotic epidermis with wide, elongated, branching rete ridges. Acral fibrokeratoma and rudimentary supernumerary digit are indistinguishable clinically. The latter however is congenital, seen at the base of little finger and histologically shows increased nerve bundles at the base of the lesion.[61],[62],[63]
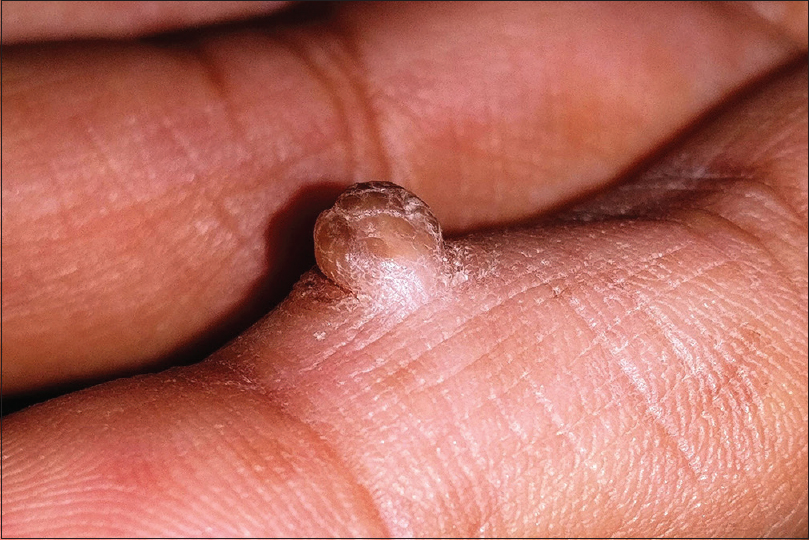 |
| Figure 11: Acral fibrokeratoma |
 |
| Figure 12: Rudimentary supernumerary digit |
 |
| Figure 13: Cutaneous horn |
Cutaneous horn
Cutaneous horn is a firm keratotic horny excrescence commonly involving the face and dorsum of the hands of elderly. The lesion may have a keratotic rim at the base [Figure - 13]. Cutaneous horn usually overlies a benign (seborrheic keratosis, verruca vulgaris), premalignant (actinic keratosis) or a malignant lesion (squamous cell carcinoma). Histopathologically, the lesions are composed of amorphous or lamellated keratin with features of the underlying disease at the base.[64],[65]
Subungual exostosis
Subungual exostosis is typically seen arising from underneath the anteromedial region of the tip of great toes in young individuals. Trauma is the most common implicated inciting factor and hence it was previously thought to be a reactive benign osteochondral outgrowth. However, it is now classified as a true neoplasm demonstrating a pathognomonic translocation t(X; 6)(q22;q13-14). It arises most frequently from the hyponychium or the lateral sulcus as a porcelain-white firm papule with surface telangiectasia and a surrounding collarette. As it enlarges, the surface becomes hyperkeratotic. The tumor is moderately painful and associated with onycholysis and predisposition to secondary infection. Radiologically, it appears as a trabeculated bony outgrowth with an enlarged distal portion covered with radiolucent fibrocartilage. Histopathologically, mature trabecular bone showing endochondral ossification with a cap of proliferating mature cartilage is seen. Simple excision is curative.[66],[67],[68],[69]
Eccrine poroma
Eccrine poroma is a benign tumor arising from the acrosyringium. Eccrine poromas are frequently seen on the palms and soles and appear as solitary sessile pinkish-red papules or nodules often surrounded by a moat-like collarette of thick skin. Multiple lesions associated with chemotherapy and bone marrow transplantation have also been described, so have been the lesions occurring at atypical sites. Histologically, they are composed of proliferating cuboidal cells with prominent nuclei and scanty cytoplasm showing ductal differentiation (showing immunohistochemical positivity for carcinoembryonic antigen). The tumor is well-circumscribed with a clear demarcation between the lesion and the adjacent normal epidermis. Eccrine poromas are frequently misdiagnosed as acral fibrokeratoma or pyogenic granulomas because of the similar clinical appearances.[70],[71],[72],[73],[74],[75]
Other Disorders
A collarette of scaly skin may also be seen in several other disorders although not as a characteristic or consistent feature. In certain conditions such a feature may be a secondary phenomenon (e.g., collarette of scales seen on removal of lesions of stucco keratosis or crusting in impetigo). [Table - 2] enlists such disorders that inconsistently or nonspecifically exhibit collarette scaling as per the same categorization in [Table - 1].[14],[42],[76],[77],[78],[79],[80],[81]
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
| 1. |
Collarette. Available from: http://www.medical-dictionary.thefreedictionary.com/collarette. [Last accessed on 2017 Mar 08].
[Google Scholar]
|
| 2. |
Weiss L. Pityriasis rosea – An erythematous eruption of internal origin. JAMA 1903;41:20-8.
[Google Scholar]
|
| 3. |
Wood GS, Reizner GT. Other papulosquamous disorders. In: Bolognia JL, Jorizzo JL, Schaffer JV, editors. Dermatology. 3rd ed. London: Elsevier; 2012. p. 157-69.
[Google Scholar]
|
| 4. |
Sterling JC. Viral infections. In: Griffiths CE, Barker J, Bleiker T, Chalmers R, Creamer D, editors. Rook's Textbook of Dermatology. 9th ed. Oxford: Wiley-Blackwell; 2016. p. 25.1-25.95.
[Google Scholar]
|
| 5. |
Drago F, Broccolo F, Zaccaria E, Malnati M, Cocuzza C, Lusso P, et al. Pregnancy outcome in patients with pityriasis rosea. J Am Acad Dermatol 2008;58:S78-83.
[Google Scholar]
|
| 6. |
James WD, Berger TG, Elston DM, Neuhaus IM. Pityriasis rosea, pityriasis rubra pilaris, and other papulosquamous and hyperkeratotic diseases. In: James WD, Berger TG, Elston DM, Neuhaus IM, editors. Andrews' Diseases of the Skin Clinical Dermatology. 12th ed. Philadelphia: Elsevier Saunders; 2016. p. 199-208.
[Google Scholar]
|
| 7. |
Miller JL. Diseases of the eccrine and apocrine sweat glands. In: Bolognia JL, Jorizzo JL, Schaffer JV, editors. Dermatology. 3rd ed. London: Elsevier; 2012. p. 587-602.
[Google Scholar]
|
| 8. |
Hausauer AK, Cohen DE. Keratolysis exfoliativa. Dermatol Online J 2015;21 (12). Available from: http://www.escholarship.org/uc/item/6mv338nx. [Last accessed on 2017 Mar 14].
[Google Scholar]
|
| 9. |
Chang YY, van der Velden J, van der Wier G, Kramer D, Diercks GF, van Geel M, et al. Keratolysis exfoliativa (dyshidrosis lamellosa sicca): A distinct peeling entity. Br J Dermatol 2012;167:1076-84.
[Google Scholar]
|
| 10. |
Inamadar AC, Ragunatha S. Inherited ichthyosis and other hereditary disorders of keratinization. In: Inamadar AC, Palit A, Ragunatha S, editors. Pediatric Dermatology. 2nd ed. New Delhi: Jaypee Brothers Medical Publishers; 2014. p. 89-111.
[Google Scholar]
|
| 11. |
Richard G, Ringpfeil F. Ichthyoses, erythrokeratodermas and related disorders. In: Bolognia JL, Jorizzo JL, Schaffer JV, editors. Dermatology. 3rd ed. London: Elsevier; 2012. p. 837-70.
[Google Scholar]
|
| 12. |
Oji V, Metze D, Traupe H. Inherited disorders of cornification. In: Griffiths CE, Barker J, Bleiker T, Chalmers R, Creamer D, editors. Rook's Textbook of Dermatology. 9th ed. Oxford: Wiley-Blackwell; 2016. p. 65.1-625.75.
[Google Scholar]
|
| 13. |
Lallas A, Zalaudek I, Argenziano G, Longo C, Moscarella E, Di Lernia V, et al. Dermoscopy in general dermatology. Dermatol Clin 2013;31:679-94, x.
[Google Scholar]
|
| 14. |
Requena L, Requena C, Cockerell CJ. Benign epidermal tumors and proliferations. In: Bolognia JL, Jorizzo JL, Schaffer JV, editors. Dermatology. 3rd ed. London: Elsevier; 2012. p. 1795-815.
[Google Scholar]
|
| 15. |
James WD, Berger TG, Elston DM, Neuhaus IM editors. Andrews' Diseases of the Skin Clinical Dermatology. 12th ed. Philadelphia: Elsevier Saunders; 2016. p. 285-318.
[Google Scholar]
|
| 16. |
Armstrong AW, Bukhalo M, Blauvelt A. A clinician's guide to the diagnosis and treatment of candidiasis in patients with psoriasis. Am J Clin Dermatol 2016;17:329-36.
[Google Scholar]
|
| 17. |
Pereira LB. Impetigo-review. An Bras Dermatol 2014;89:293-9.
[Google Scholar]
|
| 18. |
Cole C, Gazewood J. Diagnosis and treatment of impetigo. Am Fam Physician 2007;75:859-64.
[Google Scholar]
|
| 19. |
Lawrence HS, Nopper AJ. Superficial bacterial skin infections and cellulitis. In: Long SS, Pickering LK, Prober CG, editors. Principles and Practice of Pediatric Infectious Diseases. 4th ed. Edinburgh: Elsevier Saunders; 2012. p. 427-34.
[Google Scholar]
|
| 20. |
Sparling PF, Swartz MN, Musher DM, Healy BP. Clinical manifestations of syphilis. In: Holmes KK, Sparling PF, Stamm WE, Piot P, Wasserheit JN, Corey L, et al., editors. Sexually Transmitted Diseases. 4th ed. New York: The McGraw-Hill Companies; 2008. p. 661-84.
[Google Scholar]
|
| 21. |
Tan C, Zhu WY. Arthralgia and scaly rashes over the palms and the soles. Braz J Infect Dis 2016;20:505-6.
[Google Scholar]
|
| 22. |
Tognetti L, Sbano P, Fimiani M, Rubegni P. Dermoscopy of Biett's sign and differential diagnosis with annular maculo-papular rashes with scaling. Indian J Dermatol Venereol Leprol 2017;83:270-3.
[Google Scholar]
|
| 23. |
James WD, Berger TG, Elston DM, editors. Bacterial infections. In: Andrews' Diseases of the Skin Clinical Dermatology. 11th ed. Philadelphia: Elsevier Saunders; 2011. p. 247-86.
[Google Scholar]
|
| 24. |
McShane DS, Kong HH, Myers SA. Bartonella infections: Bacillary angiomatosis, cat scratch disease and bartonellosis. In: Irvine A, Hoeger P, Yan A, editors. Harper's Textbook of Pediatric Dermatology. 3rd ed. Oxford: Wiley-Blackwell; 2011. p. 58.1-58.10.
[Google Scholar]
|
| 25. |
Rapini RP. Bacterial infections. In: Barnhill RL, Crowson AN, Magro CM, Pipekorn MW, editors. Dermatopathology. 3rd ed. New York: McGraw Hill Companies, Inc.; 2010. p. 421-45.
[Google Scholar]
|
| 26. |
Berger TG, Bravo FG. Bartonellosis. In: Goldsmith LA, Katz SI, Gilchrest BA, Paller AS, Leffell DJ, Wolff K, editors. Fitzpatrick's Dermatology in General Medicine. 8th ed. New York: The McGraw-Hill Companies; 2012. p. 2201-10.
[Google Scholar]
|
| 27. |
Paller AS, Mancini A, editors. Vascular disorders of infancy and childhood. In: Hurwitz Clinical Pediatric Dermatology. 4th ed. Edinburgh: Elsevier Saunders; 2011. p. 268-302.
[Google Scholar]
|
| 28. |
Weedon D, editor. Vascular tumors. In: Weedon's Skin Pathology. 3rd ed. Edinburgh: Churchill Livingstone Elsevier; 2010. p. 887-925.
[Google Scholar]
|
| 29. |
Saw VJ, Leonard JN. Dermatoses of the eye, eyelid and eyebrows. In: Griffiths CE, Barker J, Bleiker T, Chalmers R, Creamer D, editors. Rook's Textbook of Dermatology. 9th ed. Oxford: Wiley-Blackwell; 2016. p. 109.1-109.51.
[Google Scholar]
|
| 30. |
Lowry SC. Adult Blepharitis Clinical Presentation. Available from: http://www.emedicine.medscape.com/article/1211763-clinical#b4. [Last accessed on 2017 Mar 24].
[Google Scholar]
|
| 31. |
Smolin G, Okumoto M. Staphylococcal blepharitis. Arch Ophthalmol 1977;95:812-6.
[Google Scholar]
|
| 32. |
Adya KA, Inamadar AC, Palit A. “Pitted” lesions in dermatology. Int J Dermatol 2017;56:3-17.
[Google Scholar]
|
| 33. |
Takama H, Tamada Y, Yokochi K, Ikeya T. Pitted keratolysis: A discussion of two cases in non-weight-bearing areas. Acta Derm Venereol 1998;78:225-6.
[Google Scholar]
|
| 34. |
Nordstrom KM, McGinley KJ, Cappiello L, Zechman JM, Leyden JJ. Pitted keratolysis. The role of micrococcus sedentarius. Arch Dermatol 1987;123:1320-5.
[Google Scholar]
|
| 35. |
Zaias N. Pitted and ringed keratolysis. A review and update. J Am Acad Dermatol 1982;7:787-91.
[Google Scholar]
|
| 36. |
Cohen BA, editor. Nodules and tumors. In: Pediatric Dermatology. 4th ed. Philadelphia: Elsevier Mosby; 2013. p. 126-47.
[Google Scholar]
|
| 37. |
Paller AS, Mancini A. Viral diseases of the skin. In: Paller AS, Mancini A, editors. Hurwitz Clinical Pediatric Dermatology. 4th ed. Edinburgh: Elsevier Saunders; 2011. p. 348-69.
[Google Scholar]
|
| 38. |
Jacoby RA, Ackerman AB. Is the so-called epidermal collarette formed by epidermal or adnexal epithelium? Am J Dermatopathol 1982;4:117-24.
[Google Scholar]
|
| 39. |
Adya KA, Inamadar AC. Skin of the newborn: Physiological and pathological changes. In: Gupta P, Menon PS, Ramji S, Lodha R, editors. PG Textbook of Pediatrics. 1st ed. New Delhi: Jaypee Brothers Medical Publishers; 2015. p. 2646-53.
[Google Scholar]
|
| 40. |
Ghosh S. Neonatal pustular dermatosis: An overview. Indian J Dermatol 2015;60:211.
[Google Scholar]
|
| 41. |
Trautinger F, Hönigsmann H. Subcorneal pustular dermatosis (Sneddon-Wilkinson disease). In: Goldsmith LA, Katz SI, Gilchrest BA, Paller AS, Leffell DJ, Wolff K, editors. Fitzpatrick's Dermatology in General Medicine. 8th ed. New York: The McGraw-Hill Companies; 2012. p. 383-5.
[Google Scholar]
|
| 42. |
Lipsker D, editor. Scaling. In: Clinical Examination and Differential Diagnosis of Skin Lesions. 1st ed. France: Springer-Verlag; 2013. p. 215-8.
[Google Scholar]
|
| 43. |
Gudjonsson JE, Elder JT. Psoriasis. In: Goldsmith LA, Katz SI, Gilchrest BA, Paller AS, Leffell DJ, Wolff K, editors. Fitzpatrick's Dermatology in General Medicine. 8th ed. New York: The McGraw-Hill Companies; 2012. p. 197-231.
[Google Scholar]
|
| 44. |
Park YM, Kang H, Cho BK. Annular pustular psoriasis localized to the dorsa of the feet. Acta Derm Venereol 1999;79:161-2.
[Google Scholar]
|
| 45. |
Feldmeyer L, Heidemeyer K, Yawalkar N. Acute generalized exanthematous pustulosis: Pathogenesis, genetic background, clinical variants and therapy. Int J Mol Sci 2016;17. pii: E1214.
[Google Scholar]
|
| 46. |
Sidoroff A. Acute generalized exanthematous pustulosis. Chem Immunol Allergy 2012;97:139-48.
[Google Scholar]
|
| 47. |
Sidoroff A, Halevy S, Bavinck JN, Vaillant L, Roujeau JC. Acute generalized exanthematous pustulosis (AGEP) – A clinical reaction pattern. J Cutan Pathol 2001;28:113-9.
[Google Scholar]
|
| 48. |
Horii KA, Nopper AJ. Annular erythemas. In: Irvine A, Hoeger P, Yan A, editors. Harper's Textbook of Pediatric Dermatology. 3rd ed. Oxford: Wiley-Blackwell; 2011. p. 76.1-76.8.
[Google Scholar]
|
| 49. |
Ragunatha S, Inamadar AC. Urticaria, angioedema and annular erythemas. In: Inamadar AC, Palit A, Ragunatha S, editors. Pediatric Dermatology. 2nd ed. New Delhi: Jaypee Brothers Medical Publishers; 2014. p. 451-9.
[Google Scholar]
|
| 50. |
Weyers W, Diaz-Cascajo C, Weyers I. Erythema annulare centrifugum: Results of a clinicopathologic study of 73 patients. Am J Dermatopathol 2003;25:451-62.
[Google Scholar]
|
| 51. |
Ziemer M, Eisendle K, Zelger B. New concepts on erythema annulare centrifugum: A clinical reaction pattern that does not represent a specific clinicopathological entity. Br J Dermatol 2009;160:119-26.
[Google Scholar]
|
| 52. |
Rustin M, Cerio R. Reactive inflammatory erythemas. In: Griffiths CE, Barker J, Bleiker T, Chalmers R, Creamer D, editors. Rook's Textbook of Dermatology. 9th ed. Oxford: Wiley-Blackwell; 2016. p. 47.1-47.17.
[Google Scholar]
|
| 53. |
Woo TY, Callen JP. Dermatologic effects of cancer. In: Herberman RB, editor. Influence of the Host on Tumor Development. 1st ed. Dordrecht: Kluwer Academic Publishers; 1989. p. 74-86.
[Google Scholar]
|
| 54. |
Silva JA, Mesquita Kde C, Igreja AC, Lucas IC, Freitas AF, Oliveira SM, et al. Paraneoplastic cutaneous manifestations: Concepts and updates. An Bras Dermatol 2013;88:9-22.
[Google Scholar]
|
| 55. |
Degos R, Delort J, Civatte J, Poiares Baptista A. Epidermal tumor with an unusual appearance: Clear cell acanthoma. Ann Dermatol Syphiligr (Paris) 1962;89:361-71.
[Google Scholar]
|
| 56. |
Boyd AS. Tumors of the epidermis. In: Barnhill RL, Crowson AN, Magro CM, Pipekorn MW, editors. Dermatopathology. 3rd ed. New York: McGraw Hill Companies, Inc.; 2010. p. 556-614.
[Google Scholar]
|
| 57. |
Monari P, Farisoglio C, Gualdi G, Botali G, Ungari M, Calzavara-Pinton P, et al. Multiple eruptive clear cell acanthoma. J Dermatol Case Rep 2010;4:25-7.
[Google Scholar]
|
| 58. |
Chandra BS, Rao PN. Two cases of giant pyogenic granuloma of scalp. Indian Dermatol Online J 2013;4:292-5.
[Google Scholar]
|
| 59. |
North PE, Kincannon J. Vascular neoplasm and neoplastic-like proliferations. In: Bolognia JL, Jorizzo JL, Schaffer JV, editors. Dermatology. 3rd ed. London: Elsevier; 2012. p. 1915-41.
[Google Scholar]
|
| 60. |
Zaballos P, Llambrich A, Cuéllar F, Puig S, Malvehy J. Dermoscopic findings in pyogenic granuloma. Br J Dermatol 2006;154:1108-11.
[Google Scholar]
|
| 61. |
Adya KA, Inamadar AC, Palit A. A solitary firm nodule on the palm. J Cutan Aesthet Surg 2016;9:129-31.
[Google Scholar]
|
| 62. |
Adya KA, Palit A, Inamadar AC. Keratotic papule with a collarette of skin. Indian J Dermatol 2012;57:164-5.
[Google Scholar]
|
| 63. |
Rubegni P, Poggiali S, Lamberti A, Chiantini A, De Paola M, Peccianti C, et al. Dermoscopy of acquired digital fibrokeratoma. Australas J Dermatol 2012;53:47-8.
[Google Scholar]
|
| 64. |
Weedon D, editor. Tumors of the epidermis. In: Weedon's Skin Pathology. 3rd ed. Edinburgh: Churchill Livingstone Elsevier; 2010. p. 668-708.
[Google Scholar]
|
| 65. |
Nair PA, Chaudhary AH, Mehta MJ. Actinic keratosis underlying cutaneous horn at an unusual site – A case report. Ecancermedicalscience 2013;7:376.
[Google Scholar]
|
| 66. |
Haneke E, Di Chiacchio N, Richert B, editors. Surgery of the bony phalanx. In: Nail Surgery. 1st ed. London: Informa Healthcare; 2010. p. 149-64.
[Google Scholar]
|
| 67. |
Mertens F, Möller E, Mandahl N, Picci P, Perez-Atayde AR, Samson I, et al. The t (X; 6) in subungual exostosis results in transcriptional deregulation of the gene for insulin receptor substrate 4. Int J Cancer 2011;128:487-91.
[Google Scholar]
|
| 68. |
Baran R. Periungual tissue disorders. In: Baran R, Dawber RP, Haneke E, Tosti E, Bristow I, editors. A Text Atlas of Nail Disorders Techniques in Investigation and Diagnosis. 3rd ed. London: Martin Duntiz, Taylor and Francis Group; 2003. p. 114-64.
[Google Scholar]
|
| 69. |
Weedon D, editor. Tumors of muscle, cartilage and bone. In: Weedon's Skin Pathology. 3rd ed. Edinburgh: Churchill Livingstone Elsevier; 2010. p. 858-65.
[Google Scholar]
|
| 70. |
McCalmont TH. Adnexal neoplasma. In: Bolognia JL, Jorizzo JL, Schaffer JV, editors. Dermatology. 3rd ed. London: Elsevier; 2012. p. 1829-49.
[Google Scholar]
|
| 71. |
Coulson IH, Benton EC, Ogden S. Diagnosis of skin disease. In: Griffiths CE, Barker J, Bleiker T, Chalmers R, Creamer D, editors. Rook's Textbook of Dermatology. 9th ed. Oxford: Wiley-Blackwell; 2016. p. 4.1-4.26.
[Google Scholar]
|
| 72. |
Fujii K, Aochi S, Takeshima C, Ohtsuka M, Hamada T, Asagoe K, et al. Eccrine poromatosis associated with polychemotherapy. Acta Derm Venereol 2012;92:687-90.
[Google Scholar]
|
| 73. |
Nguyen BT, Lortscher DN, Lee RA. Multiple poromas in a bone marrow transplant recipient: A case report. Dermatol Online J 2012;18:9.
[Google Scholar]
|
| 74. |
Masamatti SS, Narasimha A, Bhat A, Chowdappa V. Eccrine porocarcinoma of the scalp: A rare case report with review of literature. J Clin Diagn Res 2016;10:ED15-6.
[Google Scholar]
|
| 75. |
Bae MI, Cho TH, Shin MK, Jeong KH. An unusual clinical presentation of eccrine poroma occurring on the auricle. Indian J Dermatol 2015;60:523.
[Google Scholar]
|
| 76. |
Miller MK, Naik NS, Nousari CH, Friedman RJ, Heilman ER. Degenerative diseases and perforating disorders. In: Elder DE, Elenitsas R, Johnson LB Jr., Murphy GF, Xu X, editors. Lever's Histopathology of Skin. 10th ed. Philadelphia: Lippincott Williams & Wilkins; 2009. p. 389-405.
[Google Scholar]
|
| 77. |
Aronson IK, Fishman PM, Worobec SM. Panniculitis. In: Goldsmith LA, Katz SI, Gilchrest BA, Paller AS, Leffell DJ, Wolff K, editors. Fitzpatrick's Dermatology in General Medicine. 8th ed. New York: The McGraw-Hill Companies; 2012. p. 732-55.
[Google Scholar]
|
| 78. |
Cullen D, Díaz Recuero JL, Cullen R, Rodríguez Peralto JL, Kutzner H, Requena L, et al. Superficial acral fibromyxoma: Report of 13 cases with new immunohistochemical findings. Am J Dermatopathol 2017;39:14-22.
[Google Scholar]
|
| 79. |
Sánchez Neila N, Fonda Pascual P, Hermosa Zarza EM, García del Real CM, García de la Vega MU. A solitary hyperkeratotic papule on the palm. Indian J Dermatol Venereol Leprol 2016;82:237-8.
[Google Scholar]
|
| 80. |
Willoughby C, Soter NA. Stucco keratosis. Arch Dermatol 1972;105:859-61.
[Google Scholar]
|
| 81. |
Mervak J, Amadi U, Khandpur R, Ha Lan TT, Hristov A, Do TT, et al. Case series of volar juvenile xanthogranuloma: Clinical observation of a peripheral rim of hyperkeratosis. J Dermatol 2014;41:933-6.
[Google Scholar]
|
Fulltext Views
63,306
PDF downloads
8,716





