Translate this page into:
Diagnosis and treatment response monitoring of scabies with reflectance confocal microscopy: A diagnostic pearl
Correspondence Address:
Xavier Fust�-Novell
Department of Dermatology, Hospital Clinic de Barcelona, C/Villarroel, 170, Zip Code 08036, Barcelona
Spain
| How to cite this article: Fust�-Novell X, Morgado-Carrasco D, Alejo B, Riera-Monroig J, Puig S. Diagnosis and treatment response monitoring of scabies with reflectance confocal microscopy: A diagnostic pearl. Indian J Dermatol Venereol Leprol 2020;86:101-103 |
Problem
Scabies is a common highly contagious skin parasitosis caused by the mite Sarcoptes scabiei var. hominis. Early identification and prompt treatment of infested subjects is essential, as missed diagnosis may result in outbreaks, considerable morbidity, and significantly increased economic burden. Diagnosis is based on the clinical picture and confirmation consists of the demonstration of the mite, its eggs, or feces (scybala) in skin scrapings. Ex vivo microscopic identification of mites, ova, or scybala in skin scrapings is time-consuming and a risk-associated invasive procedure, and its sensitivity is low (46% according to some studies).[1] Dermoscopy is a simple and rapid technique that has demonstrated a high sensitivity (83%) in the diagnosis of scabies.[1] However, its specificity is lower (46%) because it does not allow visualization of eggs and feces. Moreover, the so-called “delta wing jet” sign can be difficult to differentiate from artefacts induced by scratching and bleeding and is hardly visible on dark skin or in hairy body areas, making diagnosis difficult.[1] In addition, persistent pruritus after treatment is a frequent problem in scabies and one needs to differentiate between immune-mediated pruritus and persistence of active infestation by mites. None of the two previously described techniques provide reliable indicators of the persistence of the infestation after treatment.
Solution
To perform reflectance confocal microscopy (RCM) forin vivo examination of a skin lesion clinically and/or dermoscopically compatible with a S. scabiei burrow [Figure - 1]a, [Figure - 1]b, [Figure - 1]c, [Figure - 1]d.
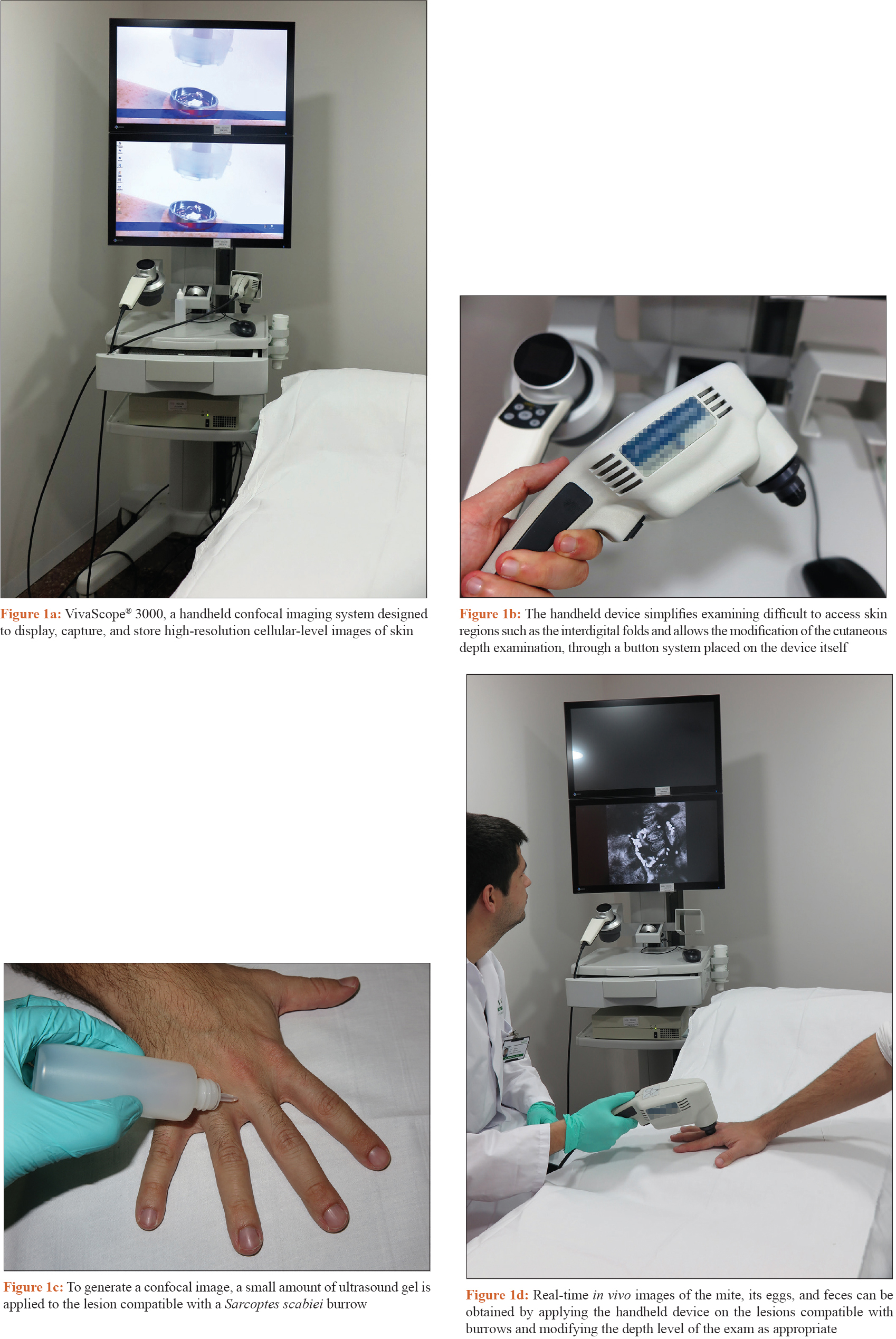 |
| Figure 1: |
RCM is a noninvasive imaging technique which allows real-time visualization of cellular components in the skin, providing serial transverse cuts at different depths. Mites, ova, and scybala can be observedin vivo and in real time using RCM [Figure - 2], enabling the clinician to confirm the diagnosis of scabies without need to perform invasive techniques.[2] According to some studies, RCM shows a specificity of 100% and a sensitivity of 92% for the diagnosis of scabies.[3]
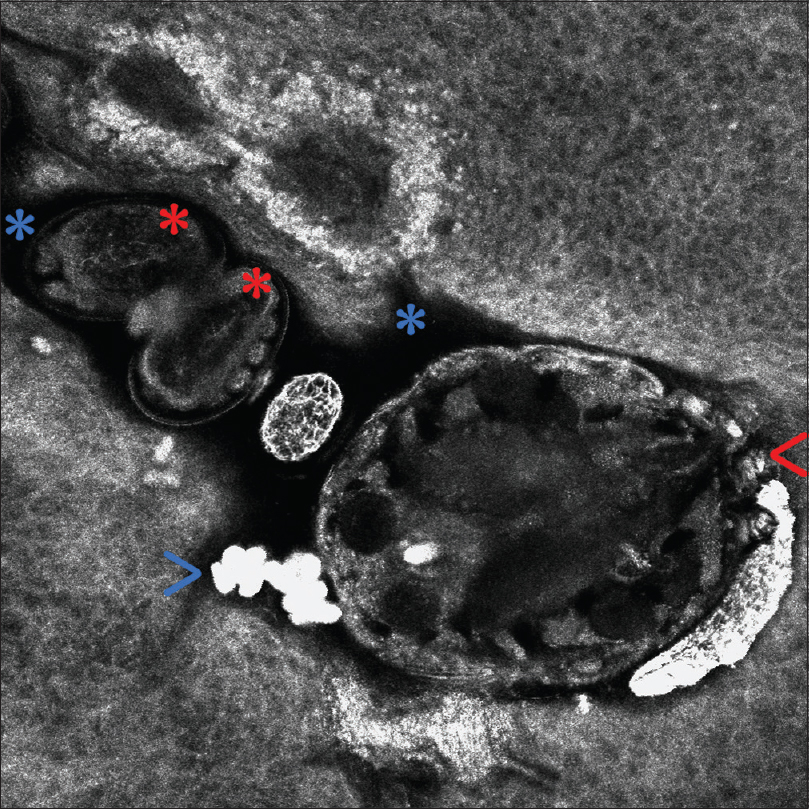 |
| Figure 2: In vivo reflectance confocal microscopy of Sarcoptes scabiei var. hominis within a burrow (VivaScope® 3000). The mite head and the anterior legs (red arrow) can be observed. Inside the hyporefractile burrow (blue asterisks), eggs containing mite embryos (red asterisks), along with ovoid hyperrefractile structures corresponding to fecal material (scybala) (blue arrow) can be seen |
Development of resistance to scabicides appears to be increasing; therefore, markers of treatment efficacy are required.[4] Traditionally, the observation of the S. scabiei mite's movements under light microscopy after a skin scraping has been considered the only way to confirm its viability in the laboratory setting. However, lack of movement of the mite in ex vivo light microscopy observation is not a reliable indicator of scabicide efficacy, as it can be due to traumatic injury to the parasite during the scraping procedure.[4] RCM is able to clearly differentiate living from dead parasites in case of posttreatment follow-up. 3 RCM observation of the mite's movements and visualization of peristalsis of the parasite gut appear to be reliable indicators of parasite viabilityin vivo and might be useful in the clinical situation to determine scabicide efficacy [Online Supplemental Video 1].[3],[4]
In conclusion, if an RCM device is available, it may be utilized as a noninvasive tool for the diagnosis of scabies. After performing a clinical and a dermoscopic examination of the skin, performance of RCM on the suspected areas is useful to confirm the presence of the parasites and to establish their viability after treatment.[3] The high cost of the RCM device is the main limiting factor of the technique. However, these devices are often available in specialized centers, due to their utility in the evaluation of pigmented lesions. In settings where the device is available, its use does not have any additional cost. The process is also time consuming and therefore this technique should be performed only in patients with doubtful lesions or with persistent pruritus after treatment.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
| 1. |
Micali G, Lacarrubba F, Verzì AE, Chosidow O, Schwartz RA. Scabies: Advances in noninvasive diagnosis. PLoS Negl Trop Dis 2016;10:e0004691.
[Google Scholar]
|
| 2. |
Lacarrubba F, Verzì AE, Micali G. Detailed analysis ofin vivo reflectance confocal microscopy for Sarcoptes scabiei hominis. Am J Med Sci 2015;350:414.
[Google Scholar]
|
| 3. |
Cinotti E, Labeille B, Cambazard F, Biron AC, Chol C, Leclerq A, et al. Videodermoscopy compared to reflectance confocal microscopy for the diagnosis of scabies. J Eur Acad Dermatol Venereol 2016;30:1573-7.
[Google Scholar]
|
| 4. |
Levi A, Mumcuoglu KY, Ingber A, Enk CD. Assessment of Sarcoptes scabiei viabilityin vivo by reflectance confocal microscopy. Lasers Med Sci 2011;26:291-2.
[Google Scholar]
|
Fulltext Views
4,369
PDF downloads
2,228





