Translate this page into:
Drug Eruptions Induced by Allopurinol Associated with HLA-B*5801
Correspondence Address:
Hong Sang
Department of Dermatology, Jinling Hospital, Medical School of Nanjing University, Nanjing, China, 210002
| How to cite this article: Zeng M, Zhang M, Liu F, Yan W, Kong Q, Sang H. Drug Eruptions Induced by Allopurinol Associated with HLA-B*5801. Indian J Dermatol Venereol Leprol 2015;81:43-45 |
Abstract
Allopurinol, a drug commonly used for treating gout and hyperuricemia, is a frequent cause of drug eruptions. Recent investigations suggest that HLA-B*5801 allele is a very strong marker for allopurinol-induced cutaneous adverse drug reactions (cADRs). In this article we report two cases of allopurinol-induced drug eruptions in patients carrying the HLA-B*5801 allele and review the literature on the association between HLA-B*5801 and allopurinol-induced cADRs based on a MEDLINE and PubMed searchINTRODUCTION
Allopurinol is a frequent cause of drug eruptions and studies have shown that patients carrying the HLA-BFNx015801 allele are at an increased risk of developing allopurinol-induced cADRs. We report two patients who presented to our department with drug eruptions temporally related to the ingestion of allopurinol which had been prescribed to treat chronic renal insufficiency. Both patients carried the HLA-BFNx015801 allele.
Case Reports
Patient 1
A 73-year-old male was admitted to our department with a 8-day history of fever and diffuse erythema all over the body. The patient had been on allopurinol and benzbromarone (both uricosuric agents) because of chronic renal insufficiency as also on vildagliptin (an antidiabetic) and calcitriol for 1 month. The problem started with moderate grade fever, a mildly itchy generalized erythematous maculopapular rash and bilateral pedal edema. The red blood cell count was 3.93 × 10 12 /L, uric acid was 540 μmol/L, urea 30.4 mmol/L, creatinine 475 μmol/L, creatine kinase (CK) 55 U/L, CK-MB 55 U/L, lactate dehydrogenase (LD) 343 U/L, and myoglobin 200 ng/ml. On the adverse drug reactions (ADRs) probability scale, [1] the score was 7.
Patient 2
A 49-year-old male was admitted with a 10-day history of fever and generalized itching and erythema. The patient had chronic renal insufficiency for the past 10 years for which he had been taking amlodipine, valsartan, and some Chinese medicines; allopurinol was added 1 month prior to presentation. Twenty days after initiation of allopurinol, the patient developed fever, facial edema, and generalized itching, erythema and scaling [Figure - 1]. On laboratory evaluation, there was leukocytosis (total white cell count, 17.6 × 10 9 /L) a red blood cell count of 3.84 × 10 12 /L, uric acid levels of 509 μmol/L, urea levels of 15.9 mmol/L, creatinine of 205 μmol/L, alanine aminotransferase (ALT) of 131U/L, and albumin of 33.6 g/L. The case got a score of 9 according to the ADRs probability scale. [1]
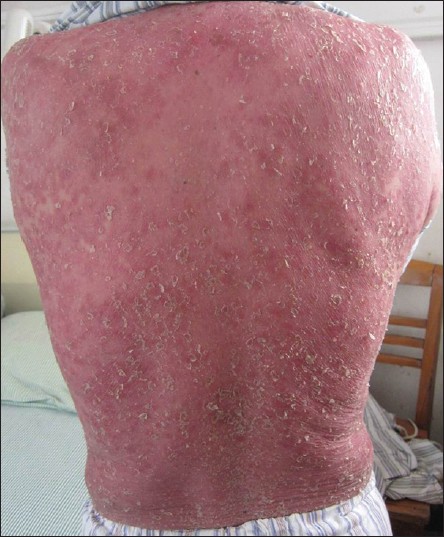 |
| Figure 1: The lesions of patient 2. Diffuse erythema and scaling on his back |
In both the patients serologic HLA-BFNx015801 typing performed by the pyrosequencing method using a PG5801 DNA detection kit (Takara-Gene, Dalian, China, D9081) was found positive. This was further confirmed by polymerase chain reaction using sequence based typing (PCR-SBT) done at Beijing Genomics Institution, Shenzhen, China [Table - 1]. The two cases were finally diagnosed as allopurinol-induced exfoliative dermatitis in patients carrying the HLA-BFNx015801 allele.

After stopping allopurinol, the patients were treated with methylprednisolone injections, 40 mg/day along with compound glycyrrhizin injection and symptomatic treatment. Both patients responded with the clinical symptoms disappearing in 1-2 weeks.
DISCUSSION
Drug eruption is an adverse drug reaction which is difficult to predict from the known pharmacology of the drug. [2] The risk for development of a drug eruption is determined by several factors including age and gender of the patient and the dose and the nature of the medication itself. Allopurinol, a commonly prescribed medication for gout and hyperuricemia, is a frequent cause of severe cutaneous adverse reactions (SCARs), which include drug hypersensitivity syndrome, Stevens-Johnson syndrome (SJS), and toxic epidermal necrolysis (TEN). [3] Recent investigations suggest that HLA-BFNx015801 is a very strong marker for allopurinol-induced cutaneous adverse drug reactions, especially severe cutaneous adverse reactions (SCARs) [4],[5],[6],[7],[8],[9],[10],[11],[12] [Table - 2]. A total of 10 studies with 219 patients with allopurinol-induced cutaneous adverse drug reactions were identified, and 193 (87.3%) of these patients were found to carry the HLA-BFNx015801 allele. The figure was even higher in patients who had developed severe cutaneous adverse reactions (SCARs). In contrast, the presence of this allele was lower (13.3%) in healthy population controls, in patients who had SCARs induced by other medications, and patients who were allopurinol tolerant. Lee et al. [4] in a study to determine the presence of HLA-BFNx015801 allele in the immediate family members of a HLA-BFNx015801 allele positive male patient who experienced allopurinol-induced Stevens-Johnson syndrome, found his brother who had taken allopurinol for 10 years for gouty arthritis to be HLA-BFNx015801 allele negative. Interestingly, eight patients who developed milder cutaneous adverse drug reactions such as erythema multiforme major or a maculopapular rash did not carry the allele. Even though our two patients manifested exfoliative dermatitis and not severe cutaneous adverse reactions (SCARs), both carried the HLA-BFNx015801 allele.

There is an ethnic variation in the frequency at which HLA-BFNx015801 allele is present in patients who develop allopurinol-induced cutaneous adverse drug reactions. Tassaneeyakul et al.[11] noted that all 27 Thai patients who developed allopurinol-induced drug reactions carried the HLA-BFNx015801 allele. Similarly, 99.1% of the 112 Chinese Han patients who developed allopurinol-induced drug reaction carried the HLA-BFNx015801 and only 1 patient who had developed erythema multiforme major did not carry the allele. In contrast, the frequency of the HLA-BFNx015801 allele in patients with allopurinol-induced cutaneous adverse drug reactions is much lower in the Europeans (55-61%) and the Japanese (40%).
There are anecdotal reports of this allele being present in patients who developed Stevens Johnson syndrome/toxic epidermal necrolysis (SJS/TEN) to other drugs as well. Kaniwa et al. [10] observed that of the five carriers of HLA-BFNx015801 allele in their 58 patients of SJS/TEN patients, 1 patient had not received allopurinol.
Based on these studies, we believe that a screening test for the HLA-BFNx015801 allele is important when patients need to take allopurinol, as it should effectively reduce the risk for allopurinol-induced cutaneous adverse drug reactions, particularly severe cutaneous adverse reactions (SCARs).
ACKNOWLEDGMENT
The authors thank Professor Guohua Zhou and Dr. Ya′nan Chu for their advice on pharmacogenomics.
| 1. |
Naranjo CA, Busto U, Sellers EM, Sandor P, Ruiz I, Roberts EA, et al. A method for estimating the probability of adverse drug reactions. Clin Pharmacol Ther 1981;30:239-45.
[Google Scholar]
|
| 2. |
Riedl MA, Casillas AM. Adverse drug reactions: Types and treatment options. Am Fam Physician 2003;68:1781-90.
[Google Scholar]
|
| 3. |
Hung SI, Chung WH, Liou LB, Chu CC, Lin M, Huang HP, et al. HLA-B*5801 allele as a genetic marker for severe cutaneous adverse reactions caused by allopurinol. Proc Natl Acad Sci U S A 2005;102:4134-9.
[Google Scholar]
|
| 4. |
Lee MH, Stocker SL, Williams KM, Day RO. HLA-B*5801 should be used to screen for risk of Stevens-Johnson syndrome in family members of Han Chinese patients commencing allopurinol therapy. J Rheumatol 2013;40:96-7.
[Google Scholar]
|
| 5. |
Cao ZH, Wei ZY, Zhu QY, Zhang JY, Yang L, Qin SY, et al. HLA-B*58:01 allele is associated with augmented risk for both mild and severe cutaneous adverse reactions induced by allopurinol in Han Chinese. Pharmacogenomics 2012;13:1193-201.
[Google Scholar]
|
| 6. |
Chiu ML, Hu M, Ng MH, Yeung CK, Chan JC, Chang MM, et al. Association between HLA-B*58:01 allele and severe cutaneous adverse reactions with allopurinol in Han Chinese in Hong Kong. Br J Dermatol 2011;167:44-9.
[Google Scholar]
|
| 7. |
Jung JW, Song WJ, Kim YS, Joo KW, Lee KW, Kim SH, et al. HLA-B58 can help the clinical decision on starting allopurinol in patients with chronic renal insufficiency. Nephrol Dial Transplant 2011;26:3567-72.
[Google Scholar]
|
| 8. |
Kang HR, Jee YK, Kim YS, Lee CH, Jung JW, Kim SH, et al. Positive and negative associations of HLA class I alleles with allopurinol-induced SCARs in Koreans. Pharmacogenet Genomics 2011;21:303-7.
[Google Scholar]
|
| 9. |
Tassaneeyakul W, Jantararoungtong T, Chen P, Lin PY, Tiamkao S, Khunarkornsiri U, et al. Strong association between HLA-B*5801 and allopurinol-induced Stevens-Johnson syndrome and toxic epidermal necrolysis in a Thai population. Pharmacogenet Genomics 2009;19:704-9.
[Google Scholar]
|
| 10. |
Kaniwa N, Saito Y, Aihara M, Matsunaga K, Tohkin M, Kurose K, et al. HLA-B locus in Japanese patients with anti-epileptics and allopurinol-related Stevens-Johnson syndrome and toxic epidermal necrolysis. Pharmacogenomics 2008;9:1617-22.
[Google Scholar]
|
| 11. |
Lonjou C, Borot N, Sekula P, Ledger N, Thomas L, Halevy S, et al. A European study of HLA-B in Stevens-Johnson syndrome and toxic epidermal necrolysis related to five high-risk drugs. Pharmacogenet Genomics 2008;18:99-107.
[Google Scholar]
|
| 12. |
Hung SI, Chung WH, Liou LB, Chu CC, Lin M, Huang HP, et al. HLA-B*5801 allele as a genetic marker for severe cutaneous adverse reactions caused by allopurinol. Proc Natl Acad Sci U S A 2005;102:4134-9.
[Google Scholar]
|
Fulltext Views
6,021
PDF downloads
2,566





