Translate this page into:
Efficacy of hemoporfin-mediated photodynamic therapy in treating Sturge–Weber syndrome associated port-wine stains: A retrospective study
Corresponding author: Prof. Weixin Fan, Department of Dermatology and Venereology, The First Affiliated Hospital of Nanjing Medical University, 300 Guangzhou Road, Nanjing, 210029, Jiangsu, China. 13327805737@189.com
-
Received: ,
Accepted: ,
How to cite this article: Huang Y, Chen B, Yang J, Bi M, Bi L, Fan W. Efficacy of hemoporfin-mediated photodynamic therapy in treating Sturge–Weber syndrome associated port-wine stains: A retrospective study. Indian J Dermatol Venereol Leprol. 2024;90:202–9. doi: 10.25259/IJDVL_1075_2022
Abstract
Background
Sturge–Weber syndrome (SWS) is a rare condition associated with a GNAQ gene mutation, which affects neural crest cells. A pulsed dye laser (PDL) is a first-line therapy for SWS, but its outcomes are worse than those in patients with port-wine stains (PWS). Photodynamic therapy (PDT) is a promising therapeutic option for PWS. However, its use for PWS associated with SWS has rarely been studied.
Aims
To investigate the therapeutic and adverse effects of photodynamic therapy in treating SWS–associated PWS.
Methods
Patients with SWS and matched patients with large size facial PWS were included in this study. Both colorimetric assessment and visual evaluation were conducted to evaluate patients’ responses to treatment.
Results
Colorimetric assessment (blanching rate) and visual evaluation (scores of colour improvement) showed that after two PDT treatments, the SWS and PWS groups had similar treatment responses (21.2% vs. 29.8%; 3.39 vs. 3.65; P = 0.18, P = 0.37). However, there was a significant difference in efficacy between patients with SWS with and without a treatment history (12.4 and 34.9%, respectively; P = 0.02), as well as between patients with lesions located on the central and lateral faces (18.5 and 36.8%, respectively; P = 0.01). Both the SWS and PWS groups experienced minor adverse effects, and the frequency of these effects was not significantly different between the two groups.
Limitation
The study was limited by a small sample size and the possibility of later onset of glaucoma. In addition, false-negative magnetic resonance imaging screening results for SWS could not be ruled out due to the young age of some participants.
Conclusion
Photodynamic therapy is a safe and effective therapeutic option for SWS–associated PWS. Patients without a treatment history, and lesions on the lateral face, responded well, demonstrating good efficacy.
Keywords
Hemoporfin
Sturge–Weber syndrome
Port-wine stains
Photodynamic therapy
Colorimeter
Plain Language Summary
Sturge–Weber syndrome (SWS) is a rare syndrome. Facial port-wine stains (PWS) are usually observed in this condition. The effects of pulsed dye laser therapy in patients with this condition are worse than those in patients with port-wine stains without SWS. Although photodynamic therapy (PDT) is a promising therapeutic option for port-wine stains, its use in the treatment of Sturge–Weber syndrome–associated port-wine stains has rarely been studied. This study comprised 23 patients with Sturge–Weber syndrome and 23 patients with port-wine stains. It aimed to evaluate the therapeutic and adverse effects of photodynamic therapy in the treatment of Sturge–Weber syndrome–associated port-wine stains. Colorimetric assessment (blanching rate) and visual evaluation (scores of colour improvement) showed that after two photodynamic therapy treatments, the Sturge–Weber syndrome and port-wine stains groups had similar treatment responses. However, patients without a treatment history, with lesions on the lateral face, responded well to the photodynamic therapy. The Sturge–Weber syndrome and port-wine stains groups experienced minor adverse effects, such as edema, scabbing, hyperpigmentation, eczema and scarring. The frequency of these effects did not significantly differ between the two groups. We demonstrated that photodynamic therapy is a safe and effective therapeutic option for Sturge–Weber syndrome–associated port-wine stains.
Introduction
Sturge–Weber syndrome (SWS) is a rare condition that affects 1 in every 20,000–50,000 infants.1–2 The syndrome is associated with a GNAQ gene mutation, which affects neural crest cells, resulting in vascular abnormalities of the cutaneous, intracranial and eyes; its clinical manifestations include intellectual disability, seizures, hemiparesis, hemianopia and glaucoma.3 Treatment for this condition is challenging, which includes not only seizure control and intraocular pressure reduction but also treatment for port-wine stains (PWS). Laser treatments are also suitable for this condition, but the outcome was worse than PWS.4–5 Oral sirolimus may be beneficial for cognitive impairments in SWS; however, PWS associated with the condition did not show significant improvement.6 Therefore, the need for effective treatment methods for SWS–associated PWS remains unfulfilled.
Photodynamic therapy (PDT) is a vascular-targeted therapeutic option for PWS in China.7 It is an effective, or even more effective treatment than pulsed dye laser (PDL) for PWS.8–11 Darkening of lesion after the therapy was rarely reported.12 However, using PDT to treat SWS–associated PWS was rarely reported.13–14 Considering that the response of SWS to PDL is different from PWS,4–5 and skin necrosis after PDT was reported in a case,14 it is necessary to investigate the therapeutic and adverse effects of PDT in SWS.
Methods
Subjects
The criteria for diagnosing Sturge–Weber syndrome (SWS) include two or three of the following abnormalities: facial port-wine stains, brain vascular malformation and eye vascular malformation.4 All patients with SWS who received at least two sessions of Photodynamic therapy (PDT) treatment in the department of dermatology, from January 2020 to December 2021, were included in the study. Patients who were lost to follow-up after the second treatment were excluded from the study. Patients with large facial port-wine stains (>40 cm2) and normal results on brain contrast-enhanced magnetic resonance imaging (MRI) and ophthalmologic examinations prior to treatment were selected as the control group, with only lesions affecting the first and/or second branch of the trigeminal nerve were included.
All participants underwent pretreatment tests, including blood cell count, renal and liver function tests, electrocardiography, brain contrast-enhanced magnetic resonance imaging and ophthalmologic examinations. This research was approved by the hospital ethics committee (KS202056), and informed consent was obtained from all participants prior to the treatment.
Therapy process
Photosensitizer hematoporphyrin monomethyl ether (HMME) (Fudan-Zhangjiang Bio-Pharmaceutical Co Ltd., China) was intravenously pumped (5.0–5.5 mg/kg) for 20 min. Then, the target treatment area was exposed to 532 nm light (Wuhan YaGe Laser Engineering Co Ltd, China) (80–90 mW/cm2) 10 min after the injection which lasted for 18–22 min. The average treatment intervals were 3.81 ± 1.12 (range: 2–6) months. The same treatment area was carefully chosen for subsequent treatment.
Clinical images collection and evaluation
At baseline and two months after the second therapy, pictures were obtained via the VISIA-CR™ system (Visia v6.4.2, Canfield Scientific Inc, USA) or camera (EOS 700D SLR digital camera, Canon Inc, Japan) [Figure 1]. The visual evaluation was performed in an independent and blinded manner by four dermatologists. Colour improvement was graded as follows: 5 = excellent (>75% improvement), 4 = good (51–75% improvement), 3 = fair (25–50% improvement), 2 = poor (<25% improvement), and 1 = no improvement.15 Results were deemed valid with the agreement of at least three dermatologists; otherwise, the evaluation was repeated.

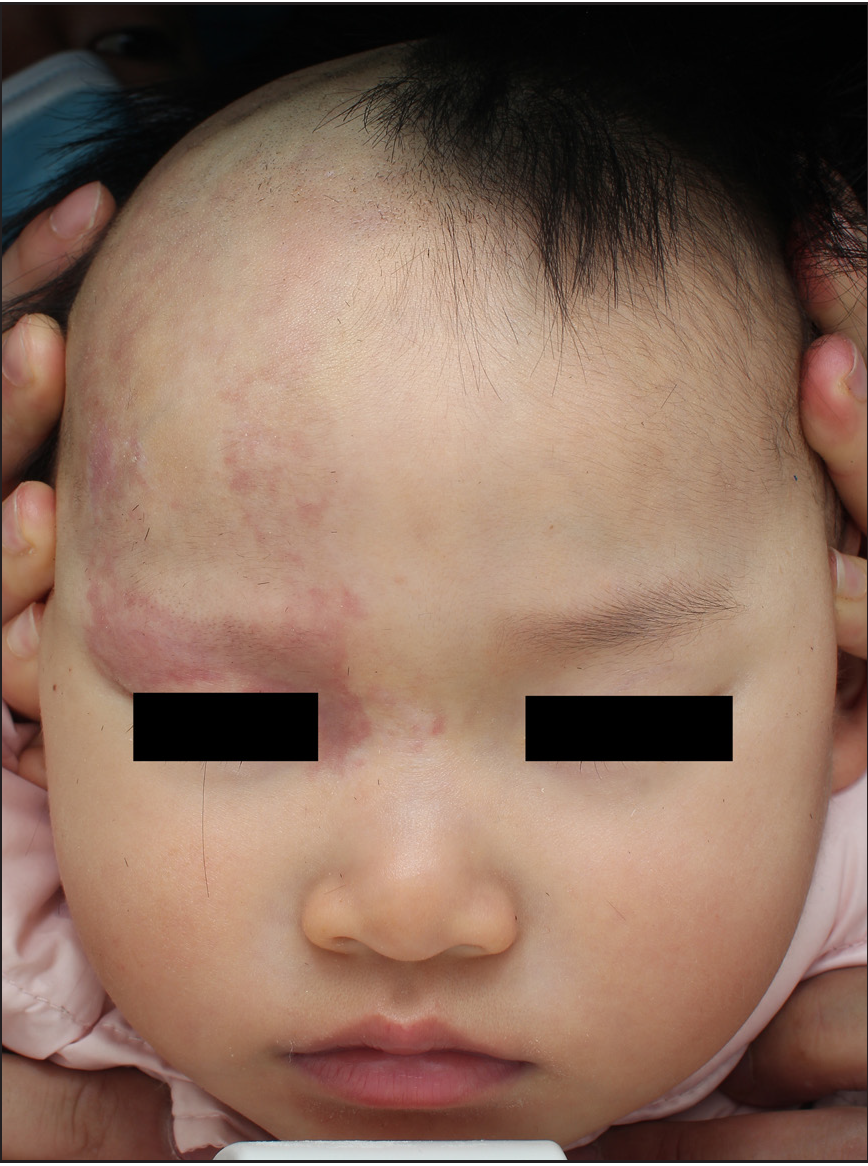
- Photodynamic therapy for patients with Sturge–Weber syndrome (SWS): A 2-year-old girl: Before treatment
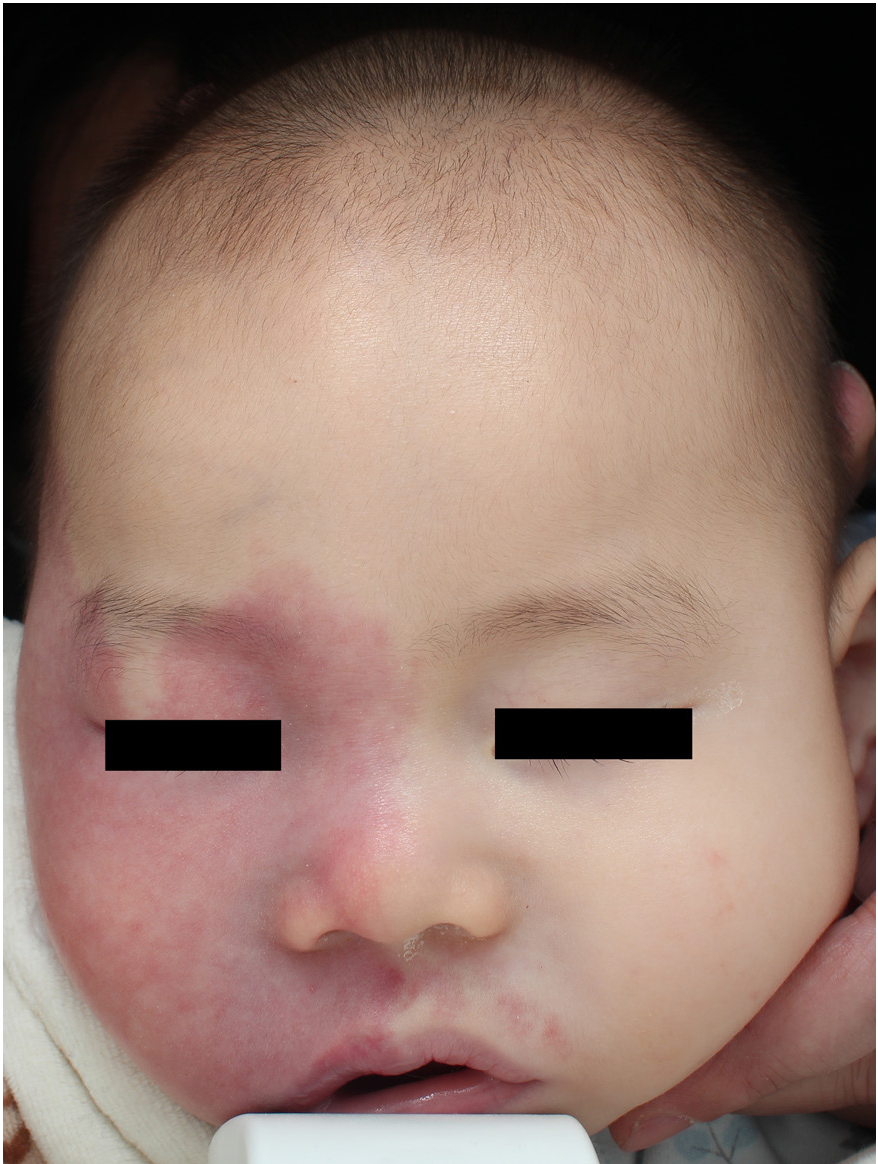
- Photodynamic therapy for patients with Sturge–Weber syndrome (SWS): A 2-year-old girl: After two treatment sessions (51–75% improvement)
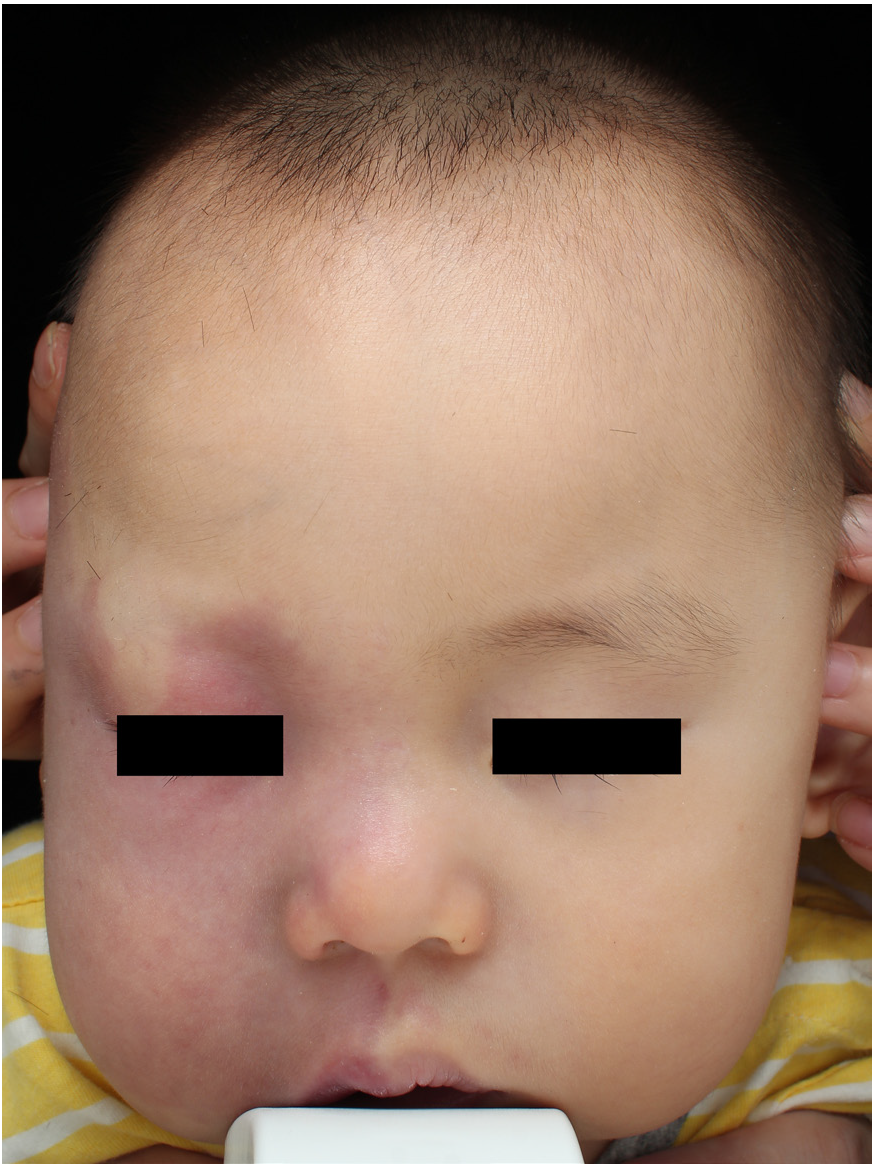
- Photodynamic therapy for patients with Sturge–Weber syndrome (SWS): A 1-year-old boy: Before treatment
- Photodynamic therapy for patients with Sturge–Weber syndrome (SWS): A 1-year-old boy: After two treatment sessions (25–50% improvement).
Colorimetrical data acquisition and analysis
The colour of representative treatment areas (3–6 sites) and their contralateral skin were recorded using a chromometer (Minolta CM-700d, Konica, Japan). Three consecutive measurements were made automatically on each site and the device displayed the mean values. The L* (lightness), a* (green to red) and b* (blue to yellow) values of each patient were calculated by the average of L*, a*, and b* values in multiple sites, respectively. The same sites of each patient were selected at every visit based on a marked photo. The colour difference was calculated according to the following equation: .16 The ΔL*, Δa* and Δb* represent different values of L*, a* and b* values between the lesion and its contralateral site, respectively. The blanching rate (%) was given by (1–ΔEafter treatment/ΔEbefore treatment) × 100 (%).16
Side effects
Follow-up analysis was conducted via smartphone on 1, 3, 7, 15 and 45 days after each treatment. The participants were asked to send photographs of the treatment area and report any abnormal symptoms such as seizures, eye pain, headache, nausea, vomiting or rapid loss of vision. All participants successfully completed the follow-up. Anticonvulsant and/or antiglaucoma therapy were maintained and appropriate care after treatment was deemed necessary.
Statistical analysis
The Student’s t-test and analysis of variance were used to calculate the blanching rate and age differences between two and three groups, respectively. Wilcoxon test was used to analyse the efficacy (colour improvement scores) differences between two groups. Spearman’s rank correlation coefficients were used to compare the results of the colorimetric assessment and visual evaluation in all participants. The frequency differences among groups were evaluated using the Chi-square test or Fisher’s exact test. All analyses were performed using SPSS 20 and P < 0.05 was considered significant.
Results
Demographic data
Twenty-seven patients with Sturge–Weber syndrome received at least two sessions of photodynamic therapy. Four patients were lost to follow-up. Finally, 23 Sturge–Weber syndrome and 23 port-wine stains patients were included. The sex ratio, age, subtype of Sturge–Weber syndrome, treatment history and Fitzpatrick skin types of the Sturge–Weber syndrome and port-wine stain groups were comparable [Table 1].
| Characteristics | SWS (N) | PWS (N) | P | |
|---|---|---|---|---|
| Total | Male | 15 | 14 | 0.76 |
| Female | 8 | 9 | ||
| Mean age | 3.43 ± 4.78 | 3.70 ± 6.17 | 0.87 | |
| Without a treatment history | Male | 6 | 6 | 1.00 |
| Female | 3 | 3 | ||
| Mean age | 1.78 ± 0.67 | 1.56 ± 0.53 | 0.44 | |
| Branch of the trigeminal nerve involved | V1 | 6 | 7 | - |
| V2 | 1 | 10 | ||
| V1 + V2 | 10 | 6 | ||
| V1 + V2 + V3 | 3 | 0 | ||
| V1 + V3 and Bilateral V2 | 1 | 0 | ||
| Bilateral V1 + V2 | 1 | 0 | ||
| Bilateral V1 + V2 + V3 | 1 | 0 | ||
| Subtype of PWS | Red | 19 | 21 | 0.56 |
| Purple | 1 | 1 | ||
| Hypertrophic | 3 | 1 | ||
| Treatments history | Yes | 14 | 14 | 1.00 |
| None | 9 | 9 | ||
| Fitzpatrick Skin Type | III | 18 | 19 | 1.00 |
| IV | 5 | 4 | ||
| Size of PWS | >40 cm2 | 23 | 23 | - |
SWS: Sturge–Weber syndrome; PWS: Port-wine stains; V1: ophthalmic branch of the trigeminal nerve; V2: maxillary branch of the trigeminal nerve; V3: mandibular branch of the trigeminal nerve.
Therapeutic responses between patients with Sturge–Weber syndrome and port-wine stains
After two photodynamic therapy treatments, the visual evaluation showed the mean scores of colour improvement, which were 3.39 (SD = 1.12) and 3.65 (SD = 0.93) [Table 2]; the colorimetric assessment showed the average blanching rates, which were 21.2 (SD = 23%) and 29.8% (SD = 19.8%) in the Sturge–Weber syndrome and port-wine stains group, respectively [Table 3]. The blanching rate, scores of colour improvement between Sturge–Weber syndrome and port-wine stains showed no significant difference (t = 1.36, P = 0.18 and Z = 0.89, P = 0.37; respectively) [Figure 2]. Besides, the blanching rates between untreated Sturge–Weber syndrome (n = 9) and port-wine stains (n = 9) also showed no significant differences (34.9% vs. 33.5%; t = 0.15, P = 0.88).
| Scores | Grade of colour improvement | SWS | PWS | ||
|---|---|---|---|---|---|
| Visual evaluation (n/%) | Colorimetric assessment (n/%) | Visual evaluation (n/%) | Colorimetric assessment (n/%) | ||
| 1 | No improvement (0) | 1 (4.3) | 5 (21.7) | 0 (0) | 1 (4.3) |
| 2 | Poor (<25%) | 3 (13.0) | 7 (30.4) | 3 (13.0) | 9 (39.1) |
| 3 | Fair (25–50%) | 10 (43.5) | 10 (43.5) | 6 (26.1) | 11 (47.8) |
| 4 | Good (51–75%) | 4 (17.4) | 1 (4.3) | 10 (43.5) | 1 (4.3) |
| 5 | Excellent (>75%) | 5 (21.7) | 0 (0) | 4 (17.4) | 1 (4.3) |
| Average Scores | 3.39 ± 1.12 | 2.30 ± 0.88 | 3.65 ± 0.93 | 2.65 ± 0.83 | |
SWS: Sturge–Weber syndrome; PWS: Port-wine stains
| Characteristic | SWS | PWS | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N (case) | No improvement (%) | Poor (%) | Fair (%) | Good (%) | Excellent (%) | Mean blanching rate (%) | P values | N (case) | No improvement (%) | Poor (%) | Fair (%) | Good (%) | Excellent (%) | Mean blanching rate (%) | ||
| Total | 23 | 5 (21.7) | 7 (30.4) | 10 (43.5) | 1 (4.3) | 0 (0) | 21.2 | 23 | 1 (4.3) | 9 (39.1) | 11 (47.8) | 1 (4.3) | 1 (4.3) | 29.8 | ||
| Gender | Male | 15 | 4 (26.7) | 4 (26.7) | 6 (40.0) | 1 (6.7) | 0 (0) | 18.9 | 0.53 | 14 | 0 (0) | 4 (28.6) | 9 (64.3) | 0 (0) | 1 (7.1) | 36.6 |
| Female | 8 | 1 (12.5) | 3 (37.5) | 4 (50.0) | 0 (0) | 0 (0) | 25.5 | 9 | 1 (11.1) | 5 (55.6) | 2 (22.2) | 1 (11.1) | 0 (0) | 19.3 | ||
| Age (years) | <3 | 17 | 3 (17.6) | 5 (29.4) | 8 (47.1) | 1 (5.9) | 0 (0) | 24.3 | 0.28 | 17 | 1 (5.9) | 6 (35.3) | 8 (47.1) | 1 (5.9) | 1 (5.9) | 31.7 |
| ≥3 | 6 | 2 (33.3) | 2 (33.3) | 2 (33.3) | 0 (0) | 0 (0) | 12.4 | 6 | 0 (0) | 3 (50.0) | 3 (50.0) | 0 (0) | 0 (0) | 24.6 | ||
| Subtype of PWS | Red | 19 | 4 (21.1) | 6 (31.6) | 9 (47.4) | 0 (0) | 0 (0) | 20.7 | 0.83 | 21 | 1 (4.8) | 7 (33.3) | 11 (52.4) | 1 (4.8) | 1 (4.8) | 32.0 |
| Purple and hypertrophic | 4 | 1 (25.0) | 1 (25.0) | 1 (25.0) | 1 (25.0) | 0 (0) | 23.5 | 2 | 0 (0) | 2 (100.0) | 0 (0) | 0 (0) | 0 (0) | 7.1 | ||
| Treatments history | Yes | 14 | 4 (28.6) | 6 (42.9) | 4 (28.6) | 0 (0) | 0 (0) | 12.4 | 0.02 | 14 | 1 (7.1) | 7 (50.0) | 5 (35.7) | 0 (0) | 1 (7.1) | 27.5 |
| None | 9 | 1 (11.1) | 1 (11.1) | 6 (66.7) | 1 (11.1) | 0 (0) | 34.9 | 9 | 2 (22.2) | 6 (66.7) | 1 (11.1) | 0 (0) | 0 (0) | 33.5 | ||
| System involving | Ocular abnormalities | 9 | 3 (33.3) | 3 (33.3) | 2 (22.2) | 1 (11.1) | 0 (0) | 16.6 | 0.58 | |||||||
| Intracranial abnormalities | 8 | 1 (12.5) | 3 (37.5) | 4 (50.0) | 0 (0) | 0 (0) | 20.1 | |||||||||
| Both of the above | 6 | 1 (16.7) | 1 (16.7) | 4 (66.7) | 0 (0) | 0 (0) | 29.5 | |||||||||
SWS: Sturge–Weber syndrome; PWS: Port-wine stains; Poor (<25% improvement); Fair (25%-50% improvement); Good (51%-75% improvement); Excellent (>75% improvement); P values: blanching rate differences between groups.
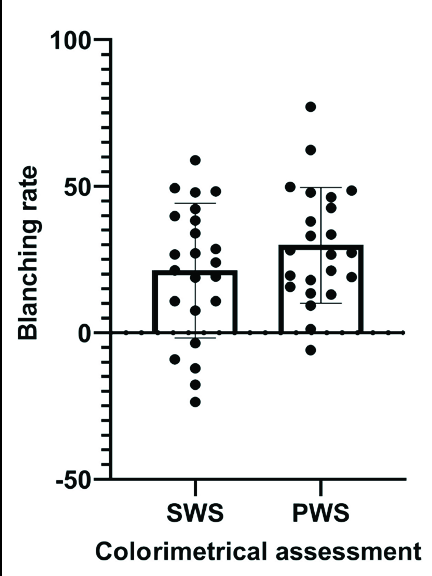
- Efficacy of photodynamic therapy in patients with Sturge–Weber syndrome (SWS) and Port-wine stains (PWS): Colorimetric assessment (shows no significant differences between SWS and PWS)
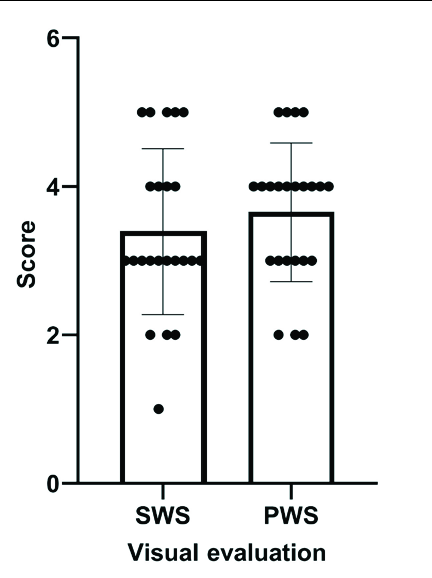
- Efficacy of photodynamic therapy in patients with Sturge–Weber syndrome (SWS) and Port-wine stains (PWS): Visual evaluation (shows no significant differences between SWS and PWS)
Therapeutic responses in different patients with Sturge–Weber syndrome
Blanching rates between patients with Sturge–Weber syndrome with and without a treatment history were significantly different after two photodynamic therapy treatments (12.4% vs. 34.9%; t = 2.57, P = 0.02) [Table 3]. But, blanching rates between types (red, and purple and hypertrophic port-wine stains), ages (<3 years or ≥3 years), sex and system involvement (ocular, intracranial or both) showed no significant differences in Sturge–Weber syndrome (t = 0.22, P = 0.83; t = 1.10, P = 0.28; t = 0.64, P = 0.53; and F = 0.56, P = 0.58, respectively).
Therapeutic responses in different locations of lesions
In 20 patients with Sturge–Weber syndrome, treatment areas with both central and lateral face lesions of port-wine stains were evaluated. To compare the therapeutic effects between the two, colorimeter values were divided into two groups for each patient (central and lateral face lesions). Following two photodynamic therapy sessions, the average blanching rate was 18.5% (SD = 26.3%) in central face lesions and 36.8% (SD = 26.8%) in lateral face lesions. The results indicated a significant difference in the blanching rate between central and lateral face lesions (t = 2.70, P = 0.01).
Side effects
In the Sturge–Weber syndrome group, side effects such as epilepsy, acute angle-closure glaucoma and photosensitivity dermatitis were not observed during the follow-up period. All treated areas (100%) developed varying degrees of edema lasting for a short term after treatment. Additionally, scabs, hyperpigmentation, eczema and scarring were observed in 23 (50%), 4 (8.7%), 1 (2.2%) and 1 (2.2%) time(s) of treatment, respectively. Hypopigmentation, pustules, serious infection and skin necrosis were not observed. Similar adverse events were observed in the port-wine stains group, but the frequency of side effects revealed no significant differences between SWS and PWS [Table 4].
| Type | SWS (N/T) | PWS (N/T) | P | |
|---|---|---|---|---|
| Systemic adverse reaction | Photosensitivity dermatitis | 0/46 | 0/46 | – |
| Epilepsy | 0/46 | 0/46 | – | |
| Acute angle-closure glaucoma | 0/46 | 0/46 | – | |
| Local adverse reaction | Edema | 46/46 | 46/46 | – |
| Scabs | 23/46 | 21/46 | 0.68 | |
| Thin scabs | 17/46 | 14/46 | 0.51 | |
| Thick scabs | 6/46 | 7/46 | 0.77 | |
| Hyperpigmentation | 4/46 | 5/46 | 1.00 | |
| Hypopigmentation | 0/46 | 1/46 | 1.00 | |
| Pustules | 0/46 | 1/46 | 1.00 | |
| Eczema | 1/46 | 0/46 | 1.00 | |
| Serious infection | 0/46 | 0/46 | – | |
| Scar | 1/46 | 0/46 | 1.00 | |
| Skin necrosis | 0/46 | 0/46 | – | |
SWS: Sturge–Weber syndrome; PWS: Port-wine stains; N: number of side effects after PDT treatment; T: total number of PDT treatments.
Discussion
PWS are characterised by ectatic capillaries in the papillary and reticular layers of the dermis. Photodynamic therapy (PDT) involves the use of a photosensitizer, light and oxygen to cause cell death through necrosis, apoptosis or autophagy by photochemical reaction. After intravenous injection, hematoporphyrin monomethyl ether is quickly absorbed by vascular endothelial cells but rarely by epidermal cells.17 During the treatment, the surrounding normal skin was covered, and the penetration of 532 nm light was limited; thus, hematoporphyrin monomethyl ether–PDT selectively destroyed the vascular endothelial cells in PWS without causing obvious damage to the epidermis.
Study has shown that PDT is a promising therapeutic option for PWS.18 In this study, the erythema of most patients with Sturge–Weber syndrome (SWS) improved after two PDT treatments. Visual evaluation revealed the average colour improvement scores were 3.39 and 3.65 in SWS and PWS groups, respectively (P = 0.37). Colorimetric assessment obtained similar results, as blanching rate between SWS and PWS also showed no significant difference (21.2% vs. 29.8%). The responses of untreated patients were analysed to eliminate the influence of previous treatment. The blanching rates were 34.9 and 33.5% in untreated SWS and PWS groups after two PDT treatments, respectively; they showed similar treatment response (P = 0.88). All the above results confirmed that PDT is an equally effective treatment for SWS, just as for PWS. Similar responses to PDT in SWS associated PWS have been observed in other studies.19 This is different from pulsed dye laser (PDL), where SWS showed worse outcomes after PDL treatment than PWS.4–5 It seems that PDT may be more effective than PDL for SWS; however, this remains to be confirmed in randomised controlled studies.
In the SWS, five patients showed negative values (–3.5, –9.1%, –12.1, –17.7 and –23.6%) in colorimetric assessment; visual evaluation confirmed that one patient had no improvement, one had poor, another had fair and two others had good improvement. In the PWS group, one patient showed negative value (–5.9%) but showed poor improvement on visual evaluation. The blanching rates were converted to colour improvement scores to determine the correlation between visual evaluation and colorimetric assessment. The results showed moderate agreement between them (coefficients = 0.41), which is different from the correlation coefficient of 0.89 reported by other scholars.16 Colorimetric assessment was significantly lower than the visual evaluation in this study (2.48 vs. 3.52) (P < 0.001). The subjective visual evaluation, hyperpigmentation after treatment and erythema improvement varied in different lesion fields, which may have led to the difference between visual and colorimetric assessments.
Stratification analysis was performed to investigate the efficacy of PDT in different populations with SWS. The results showed that patients without a prior treatment history had better PDT responses compared to those with a treatment history. Our previous research also justified a similar conclusion, which revealed that repeated treatment in the past (>5 times), showed an association with poor PDT response in facial PWS.18 The increased fibrous connective tissue of the epidermis after treatment affects the light penetration,20 which may reduce the treatment response. Studies have shown that the efficacy of PDL may be influenced by factors such as age or location of the lesion.21,22 However, the relationship between the age or location of the lesion and PDT response in PWS is not always consistent.18,23,24 In this study, the treatment area of 20 patients with SWS included both central and lateral face lesions. A self-control study showed that the blanching rate of central face lesions was significantly lower than that of lateral face lesions (18.5% vs. 36.8%, P = 0.01), confirming that PWS on the lateral face is associated with better PDT response. However, the blanching rates among patients of different ages (<3 years and ≥3 years) were not significantly different. Considering only one case of purple and three cases of hypertrophic PWS, patients with SWS were divided into two groups: red, and purple and hypertrophic PWS. Blanching rates between the two groups, and patients with different system involvement (ocular, intracranial or both) also showed no significant differences.
Studies have shown PDT to be a safe modality in the treatment of PWS.8–11,18,23,24 In this study, systemic side effects were not observed. However, most patients with SWS have undergone epilepsy or/and glaucoma ocular surgery before the PDT treatment. Some scholars concluded that the intraocular pressure may increase if PWS are obliterated.25 However, intraocular pressure was not measured in this study. We just have not received the complaints of clinical manifestations of acute angle-closure glaucoma; hence, further observations are necessary. Local adverse effects such as edema, scab formation, hyperpigmentation, eczema and scarring were observed in a few patients with SWS, but serious infection and skin necrosis were not observed. The frequency of these side effects was not significant in both the SWS and PWS and was comparable.
The study had certain limitations. The sample size was small and there was a possibility of later onset of glaucoma in some participants. In addition, false-negative magnetic resonance imaging screening results for Sturge–Weber syndrome could not be ruled out due to the young age of some participants.
To conclude, photodynamic therapy (PDT) is a safe and effective therapeutic option for Sturge–Weber syndrome (SWS)–associated port-wine stains (PWS). Patients without a treatment history and those with lesions on the lateral face, responded well, demonstrating good efficacy.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
This study was supported by the National Natural Science Foundation of China (no. 81673082 and 81972954), the Clinical Competency Enhancement Project in the People’s Hospital of Jiangsu Province (no. JSPH-MA-2021-10) and the Scientific Research Project of Wuxi Municipal Health Commission (M202245).
Conflicts of interest
There are no conflicts of interest.
References
- Update on Sturge-Weber syndrome: diagnosis, treatment, quantitative measures, and controversies. Lymphat Res Biol. 2007;5:257-64.
- [CrossRef] [PubMed] [Google Scholar]
- Sturge-Weber syndrome and port-wine stains caused by somatic mutation in GNAQ. N Engl J Med. 2013;368:1971-9.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Consensus statement for the management and treatment of port-wine birthmarks in Sturge-Weber syndrome. JAMA Dermatol. 2021;157:98-104.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Pulsed dye laser for Sturge-Weber syndrome. Arch Dis Child. 2002;87:434-5.
- [CrossRef] [PubMed] [Google Scholar]
- Sirolimus treatment in Sturge-Weber syndrome. Pediatr Neurol. 2021;115:29-40.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Twenty years of clinical experience with a new modality of vascular-targeted photodynamic therapy for port wine stains. Dermatol Surg. 2011;37:1603-10.
- [CrossRef] [PubMed] [Google Scholar]
- Side-by-side comparison of photodynamic therapy and pulsed-dye laser treatment of port-wine stain birthmarks. Br J Dermatol. 2013;168:1040-6.
- [CrossRef] [PubMed] [Google Scholar]
- Less is more: Similar efficacy in three sessions and seven sessions of pulsed dye laser treatment in infantile port-wine stain patients. Lasers Med Sci. 2018;33:1707-15.
- [CrossRef] [PubMed] [Google Scholar]
- Retrospective study of photodynamic therapy for pulsed dye laser-resistant port-wine stains. J Dermatol. 2020;47:348-55.
- [CrossRef] [PubMed] [Google Scholar]
- Efficacy of hemoporfin photodynamic therapy for pulsed dye laser-resistant facial port-wine stains in 107 children: A retrospective study. Indian J Dermatol Venereol Leprol. 2022;88:275.
- [CrossRef] [PubMed] [Google Scholar]
- Recurrence of port-wine stain after photodynamic therapy: A case report with a 22-year follow-up. Photobiomodul Photomed Laser Surg. 2021;39:795-8.
- [CrossRef] [PubMed] [Google Scholar]
- A rare case of Klippel-Trenaunay syndrome in association with Sturge-Weber syndrome treated by HMME-PDT. Photodermatol Photoimmunol Photomed. 2020;36:490-2.
- [CrossRef] [PubMed] [Google Scholar]
- Skin necrosis due to post-treatment care failure after photodynamic therapy of facial port-wine stain in Sturge-Weber syndrome - A case report. Photodiagnosis Photodyn Ther. 2021;36N:102546.
- [CrossRef] [PubMed] [Google Scholar]
- Split-face comparison of intense pulsed light with short- and long-pulsed dye lasers for the treatment of port-wine stains. Lasers Surg Med. 2010;42:720-7.
- [CrossRef] [PubMed] [Google Scholar]
- Objective evaluation of treatment effects on port-wine stains using L*a*b* color coordinates. Plast Reconstr Surg. 2001;108:842-7.
- [CrossRef] [PubMed] [Google Scholar]
- Clinical study on clinical operation and post-treatment reactions of HMME-PDT in treatment of PWS. Photodiagnosis Photodyn Ther. 2017;20:253-6.
- [CrossRef] [PubMed] [Google Scholar]
- Efficacy of influential factors in hemoporfin-mediated photodynamic therapy for facial port-wine stains. J Dermatol. 2021;48:1700-8.
- [CrossRef] [PubMed] [Google Scholar]
- Hematoporphyrin monomethyl ether photodynamic therapy for the treatment of Sturge-Weber syndrome and large segmental facial port-wine stain. Dermatol Ther. 2022;35:e15404.
- [CrossRef] [PubMed] [Google Scholar]
- Histology of port wine stains after copper vapour laser treatment. Br J Dermatol. 1989;121:217-23.
- [CrossRef] [PubMed] [Google Scholar]
- Thickness of healthy and affected skin of children with port wine stains: Potential repercussions on response to pulsed dye laser treatment. Dermatol Surg. 2004;30:1457-61.
- [CrossRef] [PubMed] [Google Scholar]
- Why do port-wine stains (PWS) on the lateral face respond better to pulsed dye laser (PDL) than those located on the central face? J Am Acad Dermatol. 2016;74:527-35.
- [CrossRef] [PubMed] [Google Scholar]
- Photodynamic therapy of port-wine stains: long-term efficacy and complication in Chinese patients. J Dermatol. 2011;38:1146-52.
- [CrossRef] [PubMed] [Google Scholar]
- Hemoporfin Photodynamic Therapy for Port-Wine Stain: A Randomized Controlled Trial. PLoS One.. 2016;11:e0156219.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Focal venous hypertension as a pathophysiologic mechanism for tissue hypertrophy, port-wine stains, the Sturge-Weber syndrome, and related disorders: proof of concept with novel hypothesis for underlying etiological cause (an American Ophthalmological Society thesis) Trans Am Ophthalmol Soc. 2013;V111N:180-215.
- [CrossRef] [PubMed] [Google Scholar]






