Translate this page into:
Efficacy of low dose intravenous immunoglobulins in children with toxic epidermal necrolysis: An open uncontrolled study
Correspondence Address:
Ranjan C Rawal
Department of Dermatology, V. S. Hospital, Ahmedabad
India
| How to cite this article: Mangla K, Rastogi S, Goyal P, Solanki RB, Rawal RC. Efficacy of low dose intravenous immunoglobulins in children with toxic epidermal necrolysis: An open uncontrolled study. Indian J Dermatol Venereol Leprol 2005;71:398-400 |
Abstract
Background: High dose intravenous immunoglobulins (IVIG) have emerged as a promising new therapy for treating the rare but potentially fatal drug reaction toxic epidermal necrolysis (TEN). Experimental in vitro studies support the view that IVIG can block the fas-fas ligand mediated apoptosis in TEN. Methods: Ten pediatric patients of TEN were treated with IVIG (0.05 - 0.1 gm/kg/day) along with antibiotics and supportive care. Results: Patients with 67% of mean body surface area of involvement showed an average of 2.1 days for arrest of progression of lesions and 8.1 days for complete reepithelization. There was no mortality. Conclusions: Low dose IVIG appears to be a safe and effective treatment for TEN in children. Randomized trials are needed to further evaluate the efficacy of IVIG and compare it with other therapeutic modalities.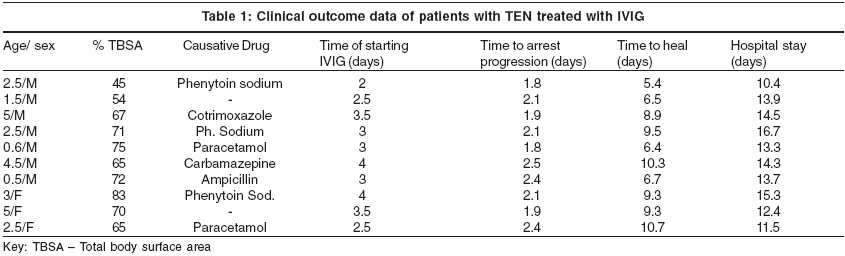

Introduction
Toxic epidermal necrolysis (TEN), also known as Lyell syndrome, is a severe and potentially life threatening form of adverse cutaneous drug reaction (ACDR).[1] TEN belongs to a category of idiosyncratic drug hypersensitivity syndromes characterized by a triad of fever, rash and variable internal organ involvement.[2] Recently, it was demonstrated that extensive keratinocyte apoptosis brought about by interactions between death receptors (CD95R/Fas) and its ligand (CD95RL/FasL) may be responsible for epidermal cell damage and separation of large areas of skin, producing scalded skin, denuded mucosa, extreme skin pain, anxiety and asthenia.[3]
Experimental evidence shows that Fas-FasL induced keratinocyte apoptosis in TEN can be blocked by intravenous immunoglobulins (IVIG).[4] On this basis and several case reports and multicenter studies on the role of IVIG in TEN,[5],[6],[7] this study was conducted to find the efficacy of low doses of IVIG treatment in patients with TEN.
METHODS
The study was conducted at the Department of Dermatology, V. S. Hospital, Ahmedabad. Clinically diagnosed cases of toxic epidermal necrolysis below the age of 12 years, during May 2003 - Nov 2003 were included in the study. The commonest offending drugs in these patients were phenytoin sodium (3 patients) followed by paracetamol (2 patients), carbamazepine, cotrimoxazole and ampicillin respectively. In 2 patients, the offending drug could not be ascertained. Total body surface area (TBSA) involvement of erythema, blistering and erosions were calculated by applying the rule of nine as in burns patients. All patients had> 30% TBSA involvement thereby excluding SJS. Baseline routine hematologic, electrolyte and blood chemistry values were determined and monitored during the treatment.
A standard TEN treatment protocol in the form of maintenance of fluid and electrolyte balance, use of non-sticky dressings (Jelonet©), potassium permanganate washings, pain control by tablet paracetamol, oral hygiene (potassium permanganate gargles, topical antifungals and oral fluconazole), diligent ophthalmic care and antibiotics according to culture sensitivity reports of wound exudates was followed in all patients. Blood cultures were performed on the day of admission to rule out infection. None of the patients′ parents gave consent for skin biopsy.
Intravenous immunoglobulin was administered in the dose of 0.05-0.1 gm/kg/day, diluted in 100 ml 5% dextrose by slow intravenous route over a time period of 1.5 - 2 hours for 5 consecutive days, only after renal creatinine clearance was determined to be normal.
RESULTS
Results were analyzed and tabulated [Table - 1]. Ten patients (7M, 3F) with an average age of 2.7 years (range: 6 months - 12 years) were seen during the 6 months period. In 3 patients, new lesions continued to appear. The average TBSA involvement was 66.7% (range: 45% -81%). The IVIG infusion was started on an average of 3.2 days. It took a mean of 2.1 days (range: 1.8 - 2.5 day) to arrest the progression of disease activity. Time taken for complete healing (re-epithelization) was 8.3 days (range: 5.4 - 10.7 day). The average duration of hospital stay was 13.6 days (range: 10.4 - 16.7 day) [Table - 1]. There was no mortality. No other systemic complications were encountered in these patients. No side effects of IVIG treatment were observed. Patients were followed up for 6 months after the treatment.
DISCUSSION
Intravenous immunoglobulin (IVIG) is a highly purified IgG prepared from pooled human plasma (more than 95% unmodified IgG) with trace amounts of IgA or IgM.[8] It is available as sterile 4.5-5.5% solution of human protein in 9-11% maltose having no preservatives and stored at 2-4 0C. IVIG is to be administered at an initial rate of 0.01 - 0.02 ml/kg body weight/minute for 30 minutes. If well tolerated, the rate may be gradually increased to maximum of 0.08 ml/kg body weight/minute. It should not be mixed with other intravenous fluids or medications. It is not compatible with saline. It may be diluted with 5% dextrose solution. It can modulate cytokine release and antibiodies present in it induce functional blockade of certain receptors and hence is useful in treating inflammatory and autoimmune disease.[9]
The exact pathogenic mechanisms involved in causation of TEN are not known. Persons with HLA-A29, -B12 and -DR7 haplotypes are more susceptible to develop SJS-TEN.[10] Differences in pharmacokinetics, metabolism and excretion of the drug, and cofactors such as infections may also play a role. The ability of an individual′s immune system to mount a predominantly Th1 (T-helper 1) or Th2 (T-helper 2) type of response may determine whether the individual will develop a simple exanthema or a severe blistering reaction such as TEN.[11] Toxic epidermal necrolysis is associated with significant morbidity, mortality and long term complications involving the eyes, skin, respiratory and gastro-intestinal systems. Several treatments such as cyclosporine,[12] cyclophosphamide,[13] plasmapheresis[14] and N-acetylcysteine[15] have shown promising results. The role of corticosteroids remains controversial, with many reports suggesting that it may even increase mortality.[16],[17]
Viard et al[4] in an in-vitro study demonstrated that extensive apoptosis is caused by interaction between the cell surface death receptor (CD95R/Fas) and its ligand (CD95RL/FasL). Both receptors and its ligand were found to be significantly overexpressed in keratinocytes of the TEN patients compared with the normal individuals. It was revealed that antibodies present in pooled purified human immunoglobulins were able to block this Fas-FasL mediated apoptosis. They treated 10 patients with TEN with 0.2 - 0.75 g/kg/d of IVIG for 4 consecutive days with favourable results.[4] In a retrospective study in pediatric population using IVIG (0.5-0.75g/kg/day x4 days) in 8 children having TEN by Tristani-Firouzi et al[12] reported favourable results with zero mortality. Our experience with IVIG in 10 pediatric patients of TEN produced similar results with no side effects. As there is no universally accepted effective treatment of TEN at present well conducted, multicentric, randomized controlled studies are needed to assess the effectiveness, safety and guidelines of use of IVIG therapy. Low dose of IVIg was given due to non-affordability of our patients. Future studies could focus on the optimum dosage for IVIg therapy for TEN[18].
| 1. |
Roujeau JC, Stern RS. Severe adverse cutaneous reactions to drugs. N Eng J Med 1994;331:1272-85.
[Google Scholar]
|
| 2. |
Sullivan JR, Shear NH. The drug hypersensitivity syndrome. What is the pathogenesis? Arch Dermatol 2001;137:357-64.
[Google Scholar]
|
| 3. |
Paul C, Wolkenstein P, Adle H, Wechsler J, Garchon HJ, Revuz J, et al. Apoptosis as a mechanism of keratinocyte death in toxic epidermal necrolysis. Br J Dermatol 1996;134:710-4.
[Google Scholar]
|
| 4. |
Viard I, Wehrli P, Bullani R, Schneider P, Holler N, Salomon D, et al. Inhibition of TEN by blockade of CD 95 with human IVIG. Science 1998;282:490-3.
[Google Scholar]
|
| 5. |
Phan TG, Wang RC. Crotty K, Adelstein S. Toxic epidermal necrolysis in AIDS treated with intravenous Immunoglobulin. Aust J Dermatol 1999;40:153-7.
[Google Scholar]
|
| 6. |
Magina S, Lisboa C, Goncalves E, Conceicao F, Leal V, Mesquita-Guimaraes J. A case of Toxic epidermal necrolysis treatment with intravenous immunoglobulins. Br J Dermatol 2000;142:191-2.
[Google Scholar]
|
| 7. |
Al-Mutairi N, Arun J, Osama NE, Amr Z, Mazen AS, Ibtesam el-A, et al. Prospective non comparative open study from Kuwait of the role of intravenous immunoglobulins in the treatment of Toxic epidermal necrolysis. Int J Dermatol 2004;43:847-51.
[Google Scholar]
|
| 8. |
Marwaha RK, Subramanian C. Intravenous immunoglobulins. Indian J Pract Pediatr 2001;4:300-4.
[Google Scholar]
|
| 9. |
Rutter A, Luger TA. High dose intravenous immunoglobulins: An approach to treat severe immune mediated and autoimmune diseases of skin. J Am Acad Dermatol 2001;44:1010-24.
[Google Scholar]
|
| 10. |
Inamder AC, Palit A. Serious cutaneous adverse drug reactions: Pathomechanism and their implications to treatment. Indian J Dermatol Venerol Leprol 2003;69:205-8.
[Google Scholar]
|
| 11. |
Breathnach SM. Drug reactions In: Champion RH, Burton JL, Burns DA, Breathnach SM, editors. Textbook of Dermatology 6th ed. London: Blackwell Science; 1998. p. 3349-518.
[Google Scholar]
|
| 12. |
Hewitt JM, Ormerod AD. Toxic epidermal necrolysis treated with cyclosporine. Clin Exp Dermatol 1992;17:264-5.
[Google Scholar]
|
| 13. |
Heng MC, Allen SG. Efficacy of cyclophosphamide in toxic epidermal necrolysis; clinical and pathophysiological aspects. J Am Acad Dermatol 1991;25:778-86.
[Google Scholar]
|
| 14. |
Kamanbroo D, Schmitz-landgraf W, Czarnetski BM. Plasmapheresis in severe drug induced toxic epidermal necrolysis. Arch Dermatol 1985;121:1548-9.
[Google Scholar]
|
| 15. |
Redondo P, de Felipe I, de la Pena A, Aramendia JM, Vanaclocha V. Drug induced hypersensitivity syndrome and toxic epidermal necrolysis: treatment with N-acetylcysteine. Br J Dermatol 1997;136:645-6.
[Google Scholar]
|
| 16. |
Sheretz EF, Jegasothy BV, Lazorus GS. Phenytoin hypersensitivity reaction presenting with toxic epidermal necrolysis and severe hepatitis: report of a patient treated with corticosteroid 'Pulse Therapy'. J Am Acad Dermatol 1985;12:178-81.
[Google Scholar]
|
| 17. |
Halebian PH, Corder VJ, Madder MR, Finklestein JL, Shires GT. Improved burn center survival of patients with toxic epidermal necrolysis without corticosteroid. Am Surg 1986;294:503-12.
[Google Scholar]
|
| 18. |
Tristani-Firouzi P, Peterson JM, Saffle JR, Morris SE, Zone JJ. Treatment of toxic epidermal necrolysis with intravenous immunoglobulins in children. J Am Acad Dermatol 2002;47:548-52.
[Google Scholar]
|
Fulltext Views
2,484
PDF downloads
2,584





