Translate this page into:
Erythema multiforme-like linear immunoglobulin A bullous dermatosis
Correspondence Address:
Hao Cheng
3 East Qingchun Road, Hangzhou, 310016
China
| How to cite this article: Han R, Sun S, Ye J, Cheng H. Erythema multiforme-like linear immunoglobulin A bullous dermatosis. Indian J Dermatol Venereol Leprol 2020;86:577-580 |
Sir,
Linear immunoglobulin A bullous dermatosis is a rare autoimmune bullous disease characterized by subepidermal blister formation and the linear and homogenous deposition of immunoglobulin A in the basement membrane zone. Herein, we report a case of proton pump inhibitor-induced linear immunoglobulin A bullous dermatosis.
A 20-year-old man (from the Hmong ethnic group in China) was admitted with a 5-day history of itchy target lesions on his abdomen and arms. He was admitted a month earlier for leptospirosis and was treated with a combination of piperacillin and tazobactam, as well as doxycycline, methylprednisolone and pantoprazole for 3 weeks. He received oral methylprednisolone (8 mg/day) along with omeprazole after being discharged. However, owing to stomach ache, he was switched from omeprazole to pantoprazole a day before the onset of the skin rash.
Laboratory investigations revealed increased serum levels of alanine aminotransferase, bilirubin, lactate dehydrogenase and immunoglobulin A. He improved following a 3-day course of methylprednisolone administered at a dose of 80 mg (1.6 mg/kg) [Figure - 1]. After receiving omeprazole for 2 days, he noticed that the rash suddenly transformed into generalized blisters [Figure - 2]. Tense vesicles and blisters filled with serous fluid were observed against an erythematous background on the patient's face, trunk and extremities and the lesions were symmetrically distributed. In some areas, the bullous lesions were clustered around a resolving lesion and appeared like a “cluster of jewels.”
 |
| Figure 1: Image showing target lesions on the upper extremity with improvement following a 3-day course of methylprednisolone |
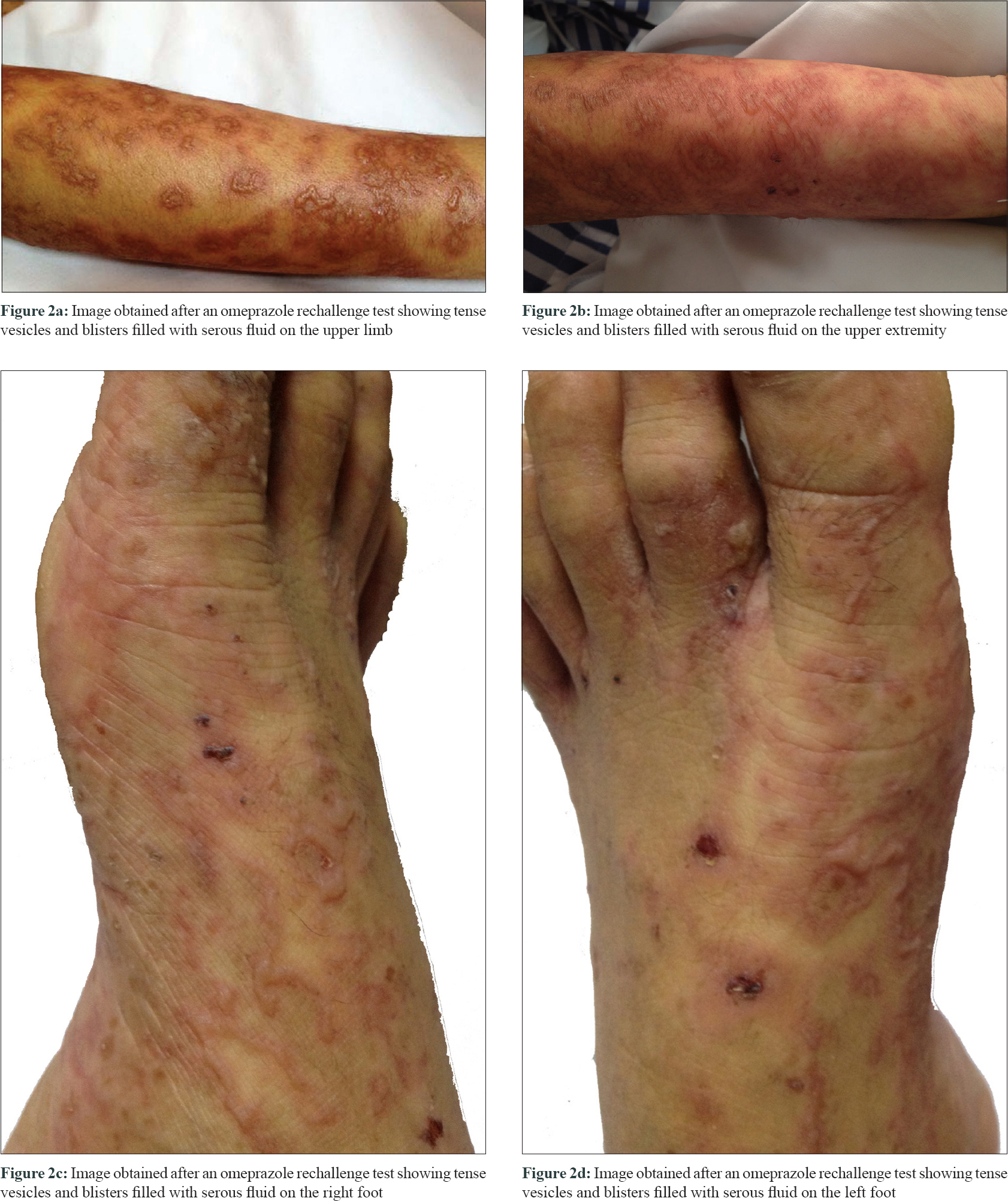 |
We suspected an allergy to omeprazole, and the drug was replaced with ranitidine. Histopathological examination of a biopsy specimen revealed a basket-weave structure of the stratum corneum, necrotic keratinocytes, full-thickness epidermal necrosis, interface change, mild lymphocytic infiltration, colloid bodies, epidermal bullae and subepidermal blisters with scattered eosinophils [Figure - 3]. Direct immunofluorescence examination revealed marked continuous linear deposition of IgA along the dermal-epidermal junction [Figure - 4]. Indirect immunofluorescence test results were negative for immunoglobulin G or immunoglobulin A at the dermal-epidermal junction, and results of an enzyme-linked immunosorbent assay for anti-desmoglein 1 and 3 were also negative. Based on these findings, he was diagnosed with drug-induced linear immunoglobulin A bullous dermatosis. Intravenous immunoglobulin therapy (400 mg/kg/day) was initiated owing to the severity and rapid deterioration in the patient's clinical condition. Development of new blisters subsided over 48 h following intravenous immunoglobulin treatment, and the patient's skin condition stabilized with evidence of re-epithelialization after the fifth intravenous immunoglobulin infusion. Complete drying and desquamation of blisters occurred after 2 weeks of treatment.
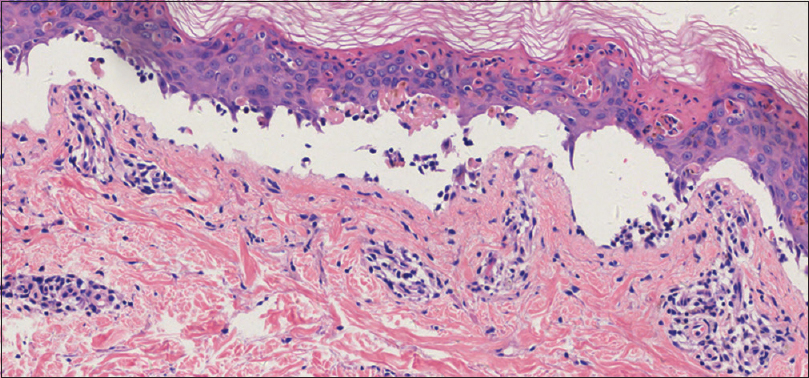 |
| Figure 3: Histopathological examination of a skin biopsy specimen from the left arm showing a basket-weave structure of the stratum corneum, necrotic keratinocytes, full-thickness epidermal necrosis, change of interface, mild lymphocytic infiltration, colloid bodies, epidermal bullae and subepidermal blisters along with scattered eosinophils, in addition to superficial perivascular lymphocytic infiltration (H and E, ×100) |
 |
| Figure 4: Direct immuno?uorescence examination of a perilesional skin biopsy specimen showing a linear band of IgA in the basement membrane zone (×400). Ig: immunoglobulin |
Previous studies have reported the following differences between idiopathic and drug-induced linear immunoglobulin A bullous dermatosis [Table - 1]. The clinical presentation of drug-induced linear immunoglobulin A bullous dermatosis is variable, and this condition may clinically and histopathologically resemble several blistering disorders including bullous pemphigoid, dermatitis herpetiformis, cicatricial pemphigoid, erythema multiforme or toxic epidermal necrolysis.[1] We initially misdiagnosed this condition as erythema multiforme; however, following the clinical presentation of tense arciform bullae in a “cluster of jewels” configuration, combined with marked continuous linear deposition of immunoglobulin A along the dermal-epidermal junction identified on direct immunofluorescence examination, we confirmed the diagnosis of linear immunoglobulin A bullous dermatosis.

Several drug classes, including antibiotics, antihypertensives and nonsteroidal anti-inflammatory agents are implicated in the development of linear immunoglobulin A bullous dermatosis. A recent review of cases of drug-induced linear immunoglobulin A bullous dermatosis reported that cutaneous manifestations occurred between 1 and 780 days after the administration of the suspected drug, and development of new lesions ceased 24–72 h after discontinuation of the offending agent.[2] In the present case, the patient received chronic treatment with a combination of piperacillin and tazobactam, as well as doxycycline, methylprednisolone and pantoprazole for 3 weeks, followed by omeprazole for 1 week before the onset of the skin rash. This was the first time the patient received pantoprazole/omeprazole. Although we cannot conclusively rule out an association between linear immunoglobulin A bullous dermatosis and the other medications administered, based on the timing and sequence of events, in this case, it is reasonable to conclude that omeprazole was the most likely contributor to linear immunoglobulin A bullous dermatosis in this patient. The piperacillin and tazobactam combination and the doxycycline were discontinued a week before the appearance of the rash, whereas omeprazole was continued until the onset of the rash and was re-administered 3 days later which was followed by the sudden development of systemic blisters, consistent with the dechallenge-rechallenge procedure.[2] Moreover, the Naranjo adverse drug reaction probability scale was 5 for the combination of piperacillin and tazobactam and doxycycline, whereas for omeprazole it was 7.
Various researchers have reported immunoglobulin E-mediated allergic reactions (particularly anaphylaxis and urticaria) to proton pump inhibitors. Concerning delayed reactions, Mockenhaupt et al. reported 9 cases of pantoprazole-induced delayed severe cutaneous reactions such as those observed in Stevens-Johnson syndrome and toxic epidermal necrolysis. A few reports of lichenoid eruption, acute interstitial nephritis, neutropenia and vasculitis attributed to proton pump inhibitors, including pantoprazole, have also been published.
A few authors have reported that infections could serve as cofactors in the immunological response contributing to the pathogenesis of drug-induced linear immunoglobulin A bullous dermatosis.[3] Triggers such as infection and subsequent treatment may initiate an immunological response, particularly in patients who are not previously sensitized to the drug.[4] The blistering eruption observed in our patient occurred 4 weeks after the diagnosis of leptospirosis. Both drugs and infection may serve as contributors to linear immunoglobulin A bullous dermatosis. The delay in skin eruption observed in our patient could be attributed to the long-term use of methylprednisolone.
Contrary to previous theories, recent studies have reported that approximately 50% of all patients with drug-induced linear immunoglobulin A bullous dermatosis require additional treatment.[2] Based on the efficacy of intravenous immunoglobulin treatment reported by previous studies, intravenous immunoglobulin was initiated in our patient to modulate the immune-mediated tissue injury which resulted in gradual symptom resolution.[5]
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form, the patient has given his consent for his images and other clinical information to be reported in the journal. The patient understands that name and initials will not be published and due efforts will be made to conceal the identity but anonymity cannot be guaranteed.
Financial support and sponsorship
This work was supported by the Foundation from the Health Bureau of Zhejiang Province (2018PY024, 2019PY037, 2018KY459), and the Fund of Chinese Traditional Medicine Administration Bureau of Zhejiang Province (2018ZQ037). The funders had no role in study design, data collection, data analysis, manuscript preparation or publication decisions.
Conflicts of interest
There are no conflicts of interest.
| 1. |
Waldman MA, Black DR, Callen JP. Vancomycin-induced linear IgA bullous disease presenting as toxic epidermal necrolysis. Clin Exp Dermatol 2004;29:633-6.
[Google Scholar]
|
| 2. |
Fortuna G, Salas-Alanis JC, Guidetti E, Marinkovich MP. A critical reappraisal of the current data on drug-induced linear immunoglobulin A bullous dermatosis: A real and separate nosological entity? J Am Acad Dermatol 2012;66:988-94.
[Google Scholar]
|
| 3. |
Baldari U, Raccagni AA, Celli B, Righini MG. Chronic bullous disease of childhood following Epstein-Barr virus seroconversion: A case report. Clin Exp Dermatol 1996;21:123-6.
[Google Scholar]
|
| 4. |
Klein PA, Callen JP. Drug-induced linear IgA bullous dermatosis after vancomycin discontinuance in a patient with renal insufficiency. J Am Acad Dermatol 2000;42:316-23.
[Google Scholar]
|
| 5. |
Mockenhaupt M, Paulmann M. Severe skin reactions due to new medications. Hautarzt 2018;69:278-89.
[Google Scholar]
|
Fulltext Views
4,793
PDF downloads
2,498





