Translate this page into:
Facial involvement in Indian psoriatic patients and its association with disease severity and metabolic syndrome: A cross-sectional study
Corresponding author: Dr. P. S. S. Ranugha, Department of Dermatology, JSS Medical College and Hospital, JSS Academy of Higher Education and Research, MG Road, Mysore - 570 004, Karnataka, India. renukaderm@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Ranugha P, Bishnoi P, Chandrashekar L. Facial involvement in Indian psoriatic patients and its association with disease severity and metabolic syndrome: A cross-sectional study. Indian J Dermatol Venereol Leprol 2021;87:522-7.
Abstract
Background:
Face was often thought to be spared in psoriasis possibly due to the protective effect of sebum and low-dose ambient ultraviolet radiation exposure. Some have suggested that facial involvement is common and indicates disease severity. There is a paucity of data on this, particularly from India. Psoriatics have a higher prevalence of metabolic syndrome, and patients with severe disease are at greater risk.
Objective:
A study of the frequency and type of facial involvement in Indian psoriatic patients and its association with disease severity and metabolic syndrome.
Methods:
A total of 250 consecutive psoriatic patients were screened and these yielded 188 patients with facial involvement. Facial psoriatics were divided into peripherofacial, centrofacial and mixed facial types. Disease severity was assessed using whole body, scalp, facial psoriasis area severity index scores and nail area psoriasis severity index scores. Patients were evaluated for the presence of metabolic syndrome using NCEP-III criteria. All parameters were compared both between facial and nonfacial psoriatics and between cases with different types of face involvement.
Results:
The mean age (P = 0.04) and age of onset of disease (P = 0.02) was lower and median whole-body psoriasis area severity index score was higher in psoriatics with facial involvement (P < 0.001) than those without. No significant association was found between facial involvement and metabolic syndrome. Mixed facial was the commonest type of facial involvement and there was a significant association of mixed facial involvement with increased total body psoriasis area severity index scores (P < 0.001).
Limitations:
Dietary habits, physical activity level, family history of diabetes and obesity were not enquired for in our patients. Centrofacial cases were too few in number, hence statistical comparisons are not relevant.
Conclusion:
Facial involvement in psoriatics is associated with severe disease but not metabolic syndrome. Mixed facial type might be considered a marker of overall psoriasis disease severity in the Indian population.
Keywords
Disease severity
facial involvement
metabolic syndrome
psoriasis
Introduction
Psoriasis is a common, chronic inflammatory disease of the skin, and facial involvement can cause considerable distress to patients. Face was often thought to be spared in psoriasis, possibly due to the protective effect of sebum and low-dose ambient ultraviolet radiation exposure.1 Recently, some studies have found that face is involved in more than 50% of psoriatic patients.2-4 Some have suggested that facial involvement may be a marker of severe psoriasis.5-7 Although centrofacial8 type was found to be associated with a higher psoriasis area severity index (PASI) and greater disease severity by some, mixed facial cases were observed to have severe disease by others.9 There is a paucity of data on facial involvement in Indian psoriatic patients, and its association with psoriasis disease severity has not been studied so far. Hence, we decided to study the clinical characteristics, frequency and type of facial involvement in Indian psoriatic patients and assess their association with disease severity.
Metabolic syndrome is a complex disorder defined by a cluster of interconnected factors that increase the risk of cardiovascular atherosclerotic diseases and diabetes mellitus type 2. It is generally defined by the presence of a combination of central obesity, hypertension, insulin resistance and dyslipidemia.10 Psoriasis is increasingly being recognized as a systemic inflammatory disorder rather than a skin disease. Several observational studies and systematic reviews have concluded that psoriatics have a greater prevalence of metabolic syndrome as well as its individual components, when compared to the general population.11-13 Patients with more severe psoriasis have greater odds of metabolic syndrome than those with milder psoriasis.12,13 Because facial involvement has been found to be associated with increased disease severity, we intended to see if facial involvement is also associated with a higher risk of metabolic syndrome.
Methods
All psoriatic patients attending the dermatology outpatient department of Jawaharlal Institute of Postgraduate Medical Education and Research Hospital, Puducherry between August 2011 and December 2012 were enrolled after Institutional Ethical Committee approval. The diagnosis was made independently by two dermatologists. Doubtful cases were biopsied and those with psoriasiform changes on histopathology were excluded. Patients with isolated scalp or palmoplantar involvement and those who were not able to give a proper history were also excluded. A detailed history regarding age of onset of the disease, age of onset of facial involvement, nail and joint involvement, associated systemic diseases such as diabetes, hypertension and hyperlipidemia and family history of psoriasis was taken. All patients were asked about the severity of pruritus and aggravating factors such as light, infection, stress, seasonal change and drug intake. Treatment history, including history of phototherapy (PUVA, ultraviolet B), systemic drugs intake (e.g. methotrexate, retinoids, cyclosporine, biologicals) and number of hospital admissions, was asked for from every patient. The treatment and admission details were verified with the patients’ hospital records, wherever possible.
An elaborate clinical examination was done for every patient. The study subjects were divided into two groups depending on the presence or absence of facial involvement. The involved facial areas were separated as follows: upper aspect of forehead (adjacent to hair line), lower aspect of forehead (separate from hair line), eyelid, cheek, malar area, nasolabial fold, perioral area, nose, periauricular area and earlobe (including external meatus). Patients were categorized into three types according to facial lesion distribution: peripherofacial type (upper forehead and/or periauricular lesions) peripherofacial, centrofacial and mixed facial type as described by Woo et al.8 The severity of psoriasis on the whole body, scalp and on the face was evaluated separately using the psoriasis area severity index.14 The severity of nail involvement was graded according to the nail area psoriasis severity index.15 The type of joint involvement and presence of pain or deformity was noted. Blood pressure was checked using sphygmomanometer and waist circumference measured using inch tape at the level of iliac crest. Fasting blood sugar and fasting lipid profile was done for all patients.
Evaluation for the presence of metabolic syndrome was done using NCEP-III criteria, with a waist circumference cut-off, as suggested by American Heart Association for Asian Americans.16,17 Patients were categorized as having metabolic syndrome if they had at least three of these five conditions: (1) waist circumference 90 cm (35 inches) or greater in men or 80 cm (32 inches) or greater in women (abdominal obesity); (2) triglycerides 150 mg/dL or higher (or receiving drug therapy for hypertriglyceridemia); (3) high-density lipoprotein-cholesterol complex <40 mg/dL in men or <50 mg/dL in women (or receiving drug therapy for reduced high-density lipoprotein-cholesterol); (4) blood pressure 130/85 mm Hg or higher (or receiving drug therapy for hypertension) and (5) fasting glucose 100 mg/dL or greater (or receiving drug therapy for hyperglycemia).
The data were collected and entered in Statistical Package for the Social Sciences version 21. Categorical variables are presented as proportion with range. Continuous variables are presented as mean with standard deviation or median and interquartile range depending on the distribution. Chi-square test was used to analyze categorical variables. Normally distributed (t-test and analysis of variance) and non-normally distributed continuous variables (Mann–Whitney and Kruskal–Wallis test) were also analyzed at a significance level of 5%. P value < 0.05 was considered significant.
Results
We recruited 250 patients for the study, of which 188 (75%) had facial involvement. Maximum number of cases were between 41 and 50 years, 74 (29.6%), followed by an equal number in 31–40 and 51–60 age groups, 46 (18.4% each). Females were more common with a male/female ratio of 1:2.73. The demographic data of the study groups are tabulated in Table 1. The mean age was found to be 43.8 and 48.3 years in patients with and without facial involvement, respectively (P = 0.04). Patients with facial involvement had an earlier onset of the disease (P = 0.02), although the difference in the mean duration of the disease between the two groups was not statistically significant (P = 0.2). The sex ratio and family history positivity were almost similar in facial and nonfacial psoriatics.
| Variable | Facial involvement | P | |
|---|---|---|---|
| No | Yes | ||
| Number of cases (%) | 62 (24.8) | 188 (75.2) | |
| Age (years), mean±SD | 48.3±15.3 | 43.8±14.4 | 0.04 |
| Sex | |||
| Male | 17 (27.4) | 50 (26.6) | 0.9 |
| Female | 45 (72.6) | 138 (73.4) | |
| Family history | |||
| No | 56 (91.8) | 173 (92.0) | 0.2 |
| Yes | 5 (8.2) | 15 (8.0) | |
| Age of onset | 39.6±0.6 | 34.7±13.915 | 0.02 |
| Duration of skin disease (years) | 8.6±9.0 | 8.9±7.6 | 0.9 |
| Worse by (%) | |||
| Seasonal change | 20 (32.3) | 59 (31.4) | 0.7 |
| Stress | 16 (25.8) | 72 (38.3) | 0.07 |
| Infection | 7 (11.3) | 15 (8) | 0.4 |
| Trauma | 5 (8.1) | 12 (6.4) | 0.6 |
SD: Standard deviation
As psoriasis area severity index scores were not normally distributed in our study population, they have been expressed as median with interquartile range. The median psoriasis area severity index score was 8.3 and 4.7 in patients with and without facial involvement, respectively (P = 0.001). A similar observation was made, when head psoriasis area severity index was compared between the two groups (P = 0.00). Systemic therapy or phototherapy was required in 81.4% (n = 153) and 61.2% (n = 38) of facial and nonfacial cases, respectively (P = 0.01) [Table 2]. Children formed a meagre group of the study population (six cases were <18 years of age). As there are no universal criteria for diagnosis of metabolic syndrome in this age group and the predictive value and utility of metabolic syndrome also has not been established, children were not screened for metabolic syndrome. Fasting lipid profile was not done for 41 patients as they were not willing or could not afford the test. Hence, metabolic syndrome screening was done for 203/250 patients. Obesity (47 vs. 60.7%, P = 0.06) and smoking (25.7 vs. 39.3%, P = 0.04) were more common in nonfacial than facial psoriatics. The difference in presence of diabetes mellitus (18% facial vs. 17.5% in nonfacial, P = 0.8) and hypertension (6.5 vs. 15.6%, P = 0.07) was not found to be statistically significant between facial and nonfacial psoriatics. We also did not find any significant difference in the presence of hypertriglyceridemia (46.9 vs. 33.1%, P = 0.07), low high-density lipoprotein (68 vs. 66.7%, P = 0.07) or metabolic syndrome (30.6% in facial vs. 27.2% in nonfacial, P = 0.2) between the two groups of patients. After controlling for smoking, the difference in obesity (P = 0.203), hypertension (P = 0.286), diabetes (P = 0.589), hypertriglyceridemia (P = 0.188), low high-density lipoprotein (P = 0.938) and metabolic syndrome (P = 0.392) was not found to be statistically significant between those with and without facial involvement.
| Variables of disease activity | Facial involvement | P | |
|---|---|---|---|
| No (n=62) | Yes (n=188) | ||
| Total PASI score-median (range) | 4.70 (2.4-7.0) | 8.3 (3.9-17.60) | 0.001 |
| Head PASI score | 0.2 (0.00-0.75) | 0.60 (0.3-1.5) | <0.00001 |
| Presence of nail involvement, n (%) | 52 (83.9) | 154 (81.9) | 0.7 |
| NAPSI score | 30.0 (12.5-50) | 30.0 (16-53) | |
| Psoriatic arthritis, n (%) | 20 (32.3) | 59 (31.4) | 0.9 |
| Treatment history, n (%) | |||
| Topical therapy | 22 (36.7) | 35 (18.6) | 0.01 |
| Systemic therapy/phototherapy | 38 (61.2) | 153 (81.4) | |
| Admission history, n (%) | 26 (41.9) | 83 (44.1) | 0.9 |
| History of erythroderma | 11 (17.7) | 42 (22.5) | 0.6 |
| Severe pruritus, n (%) | 5 (8.1) | 30 (16) | 0.2 |
PASI: Psoriasis area severity index, NAPSI: Nail area psoriasis severity index
Mixed facial [98 (52.1%)] and peripherofacial involvement [83 (44.1%)] were found to be more common, while centrofacial involvement was seen only in seven patients (3.7%) [Figures 1 and 2]. The clinical characteristics, precipitating factors and disease severity scores in the three different facial groups are tabulated in Table 3. The mean age was 41, 44.1 and 47.2 years in mixed facial, centrofacial and peripherofacial patients, respectively (P = 0.014). Duration of disease (P = 0.07), age of onset of facial involvement (P = 0.005) and duration of facial involvement (P = 0.072) were longer in peripherofacial and mixed facial than centrofacial. The mean age and age of onset of facial involvement was lower in mixed facial than peripherofacial cases (P = 0.008). However, the difference in total psoriasis area severity index alone was statistically significant between the three groups (P < 0.001) [Figure 3]. We also did not find any significant difference in facial disease severity (facial psoriasis area severity index, P = 0.06) among the three groups. Both median total psoriasis area severity index [12.3 (4.2–21.9)] and head psoriasis area severity index [0.7 (0.3–2)] scores were more in mixed facial than peripherofacial cases (P < 0.0001 for total psoriasis area severity index, P = 0.016 for head psoriasis area severity index). Cases with peripherofacial involvement showed a higher incidence of metabolic syndrome (22, 34.4%) than mixed facial patients (20, 22.7%) (P = 0.2). The difference in metabolic syndrome (0.2) and its components, viz., obesity (0.09), diabetes (0.07), hypertension (0.5), hypertriglyceridemia (0.7), low high-density lipoprotein (0.08) was not statistically significant between mixed facial, centrofacial and peripherofacial patients. Age (P = 0.082) and positive smoking history (P = 0.4) difference between the three facial groups was not statistically significant, hence adjustment for age and smoking was not done.
| Type of facial involvement (total, n=188) | P | |||
|---|---|---|---|---|
| CF (n=7; 3.7%) | MF (n=98; 52.1%) | PF (n=83; 44.1%) | ||
| Age (years), mean±SD | 44.1±13.5 | 41.0±14.5 | 47.2±13.6 | 0.014 |
| Duration of skin disease (years), mean±SD | 2.9±2.69 | 9.95±8.9 | 8.93±6.96 | 0.07 |
| Duration of facial involvement | 2.11±2.43 | 7.31±6.5 | 5.75±5.49 | 0.072 |
| Age of onset of facial involvement | 40.92±15.59 | 31.45±13.3 | 37.32±13.72 | 0.005 |
| Positive family history | 0 | 11(11.2) | 4 (4.8) | 0.2 |
| Sex | ||||
| Female | 1 (14.3) | 25 (25.5) | 24 (28.9) | 0.6 |
| Male | 6 (85.7) | 73 (74.5) | 59 (71.1) | |
| Treatment details | ||||
| Only topical therapy | 3 (42.9) | 14 (14.3) | 18 (21.7) | 0.2 |
| Systemic therapy or phototherapy | 4 (57.1) | 84 (85.7) | 65 (78.3) | |
| History of admissions in the past | ||||
| Yes | 3 (42.9) | 47 (48) | 33 (39.8) | 0.4 |
| Erythroderma in the past | ||||
| Yes | 1 (14.3) | 23 (23.7) | 18 (21.7) | 0.8 |
| Psoriatic arthritis | ||||
| Yes | 2 (28.6) | 36 (36.7) | 21 (25.3) | 0.3 |
| Metabolic syndrome | ||||
| Yes | 1/6 (16.7) | 19/89 (22.6) | 22/64 (34.4) | 0.2 |
| Severity of disease | ||||
| Total PASI** | 4.3 (1.5-8.4) | 12.3 (4.2-21.9) | 5.8 (3.2-11.9) | <0.001 |
| Head PASI | 0.3 (0.2-1.8) | 0.7 (0.3-2.0) | 0.5 (0.2-1.2) | 0.08 |
| Facial PASI | 0.3 (0.1-0.3) | 0.3 (0.2-0.6) | 0.3 (0.2-0.4) | 0.06 |
| NAPSI | 20 (20-27) | 35 (20-59) | 28.5 (14-48) | 0.4 |
SD: Standard deviation, CF: Centrofacial, MF: Mixed facial, PF: Peripherofacial, PASI: Psoriasis area severity index, NAPSI: Nail area psoriasis severity index

- Mixed facial involvement in a woman with psoriatic lesions in the ears, hairline and discrete plaques on the cheeks and forehead
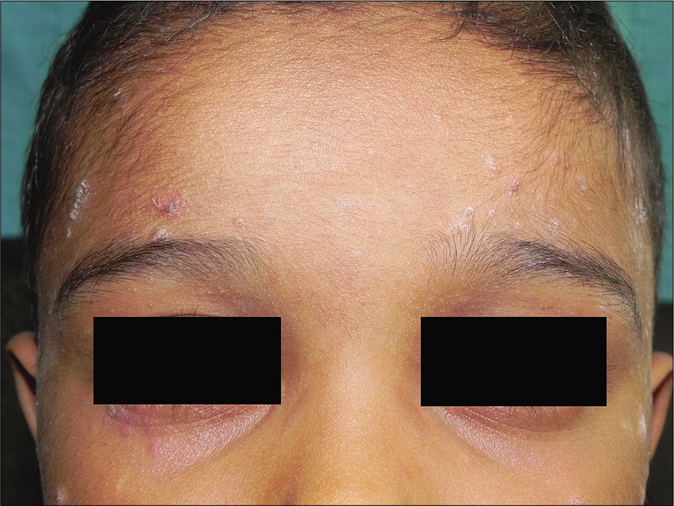
- Centrofacial involvement in a child with guttate lesions on the forehead and cheeks
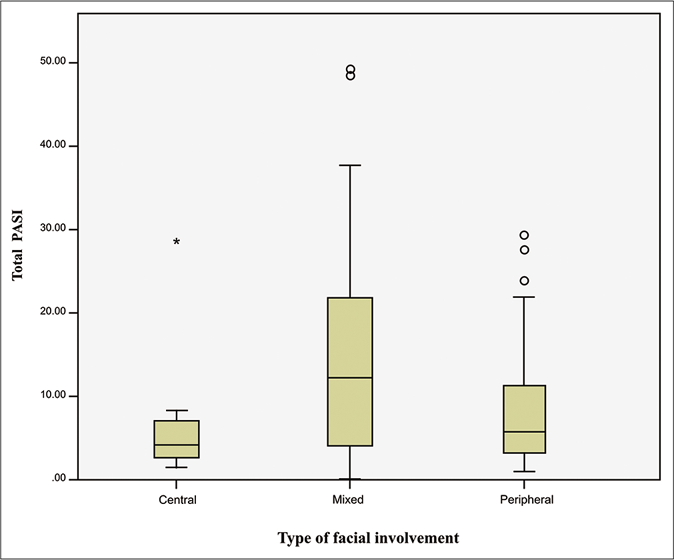
- Box plot representation of total psoriasis area severity index scores in patients with different types of facial involvement
Discussion
Although psoriasis usually has a bimodal age distribution with equal sex incidence, we found a female preponderance (2.73:1) with two-thirds of the cases between 30 and 60 years of age.
Nail psoriasis is estimated to occur in 50% of psoriatics and, in the presence of joint involvement, it can reach 80%.18 We found nail involvement in 206 (82.4%) patients. Psoriatic arthritis occurs in 30% patients with psoriasis, and in our study population, 79 (31.6%) had arthritis.19 Abdominal obesity was present in 49.2% of study subjects (60.6 and 47% among non-facial and facial psoriatics, respectively). Obesity is an important risk factor for psoriasis. Although obesity likely predates or co-exists with psoriasis, a slightly increased risk of developing obesity has been reported in patients with existing psoriasis compared with controls.20 Obesity is a risk factor for incident psoriasis, aggravates existing psoriasis and is associated with a decreased response to medical therapies.21 Metabolic syndrome was found in 58/203 patients (28.6%), which is comparable to observations made in a recent study in psoriatics from South India (28.8%),22 China (14.3%)23 and UK (34%).24
Facial involvement was seen in 75.2% of our cases, which is similar to that reported in Korea4 (67.8%), but higher than that observed in Malaysian9 (48.4%) and Iranian patients (55 and 58.7%).5,25 The higher frequency of facial involvement in our study might be due to racial differences in the study population and also because, ours is a tertiary care center, catering to a higher number of patients with moderate to severe disease. Mean age and age of onset of disease was significantly lower in psoriatics with facial involvement than those without. Similar to that observed by others,4,25,26 male gender was significantly associated with facial psoriasis by some,5,9 whereas no gender predilection was seen by others,4,25 similar to our finding. Family history positivity was found to be associated with facial involvement by some,4,25 whereas no association was found by others,9 as in our study. We observed stress-induced exacerbation and severe pruritus in a higher number of facial psoriatics, however, this was not statistically significant. Seasonal exacerbation, koebnerization and severe pruritus were found to have a significant association with facial involvement in a Korean study.4 Young Park et al.4 and Syed Nong Chek et al.9 reported nail but not joint involvement to be more common in psoriatics who had face involvement. Nail and joint involvement frequency was not different in both the groups in our study, similar to that reported by Canpolat et al.26 We found significantly higher median total psoriasis area severity index and head psoriasis area severity index scores in facial psoriatics, which is consistent with other studies.4,5,9,26 Although patients who required systemic therapy or phototherapy were more in our facial group, similar to observations by some,4,8,9 admission history was not different in the two groups in our study in contrast to others.4,9 The increased disease severity in facial psoriatics might explain the need for intensive therapy.
Smoking (25.7%) but not alcoholism was observed to have a significant association with facial involvement by us. Hypertension (adjusted odds ratio 2.6, P = 0.07) and hypertriglyceridemia (adjusted odds ratio 2.6, P = 0.07) were found to be more common in facial and nonfacial psoriatics, respectively, in our study. The association of facial involvement with metabolic syndrome has not been studied so far. No significant association of comorbidities such as diabetes mellitus, obesity or metabolic syndrome with facial involvement was found by us. The risk for metabolic syndrome and its components increases with increasing age.27 Although age difference between the groups was statistically significant (P = 0.047), it was not clinically significant, hence age adjustment for metabolic syndrome and its components was not done. We also did not find a significant association of facial involvement with metabolic syndrome after controlling for smoking.
The peripherofacial and mixed facial types were common in our study similar to Woo et al.8 However, centrofacial patients constituted at least one-fourth of their study subjects, while we had only seven centrofacial cases. The centrofacial patients were more in number than peripherofacial in an Iranian study, with mixed facial cases constituting half their study population.25 The scarcity of patients with isolated facial lesions without any scalp involvement (centrofacial) in our study might be due to racial differences in the study population. We do not have any Indian data on this for comparison. The mixed facial psoriatics had a longer duration of skin and facial disease with a significantly earlier age of onset of facial involvement (P = 0.0) compared to peripherofacial and centrofacial cases. No significant association of metabolic syndrome, nail and joint involvement or positive family history was found with any of the facial types. Interestingly, arthritis and geographic tongue were more common in centrofacial psoriatics in an Iranian study.25
Median whole body and scalp psoriasis area severity index scores were significantly higher in the mixed facial than peripherofacial group, but whole-body psoriasis area severity index scores alone were significantly higher in mixed facial when all the three groups were compared. Keshavarz et al. group found higher whole-body and scalp psoriasis area severity index scores in mixed facial, despite having a sizable number of centrofacial cases.25 Woo et al., on the other hand, found higher scalp psoriasis area severity index in peripherofacial and higher facial and whole-body psoriasis area severity index in centrofacial cases.8 We did not find any association of scalp disease severity with peripherofacial involvement and, the number of centrofacial patients were very few for comparison. Kim et al. concluded that peripherofacial psoriasis is closely associated with spreading of scalp lesions into the face rather than reflecting disease severity. No significant association of facial or nail disease severity with any of the three groups was found by us.
Limitations
Dietary habits, physical activity level, family history of diabetes and obesity, which are also risk factors for metabolic syndrome, were not enquired for in our patients. Centrofacial cases were too few in number, so disease severity comparison with other two facial types is not statistically relevant.
Conclusion
Facial involvement was associated with a lower mean age, earlier onset and increased psoriasis disease severity (both scalp and body), as has been observed earlier. Nail, joint disease and metabolic syndrome were not associated with facial involvement. Mixed facial type was associated with increased overall disease severity and might be a marker of psoriasis disease severity in the Indian population. Large-scale studies are needed to confirm this.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form the patients have given their consent for their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- Facial psoriasis: Comparison of patients with and without facial involvement. J Am Acad Dermatol. 2004;50:582-4.
- [CrossRef] [Google Scholar]
- Facial involvement in Iranian psoriatic patients and its relation to severity of the disease. J Eur Acad Dermatol Venereol. 2010;24:1488-9.
- [CrossRef] [PubMed] [Google Scholar]
- Facial involvement is a sign of severe psoriasis In: Farber EM, Nall L, Morhenn V, eds. Psoriasis: Proceedings of the Fourth International Symposium. New York: Elsevier; 1987. p. :405-6.
- [Google Scholar]
- Classification of facial psoriasis based on the distributions of facial lesions. J Am Acad Dermatol. 2008;58:959-63.
- [CrossRef] [PubMed] [Google Scholar]
- Clinical characteristics of patients with facial psoriasis in Malaysia. Int J Dermatol. 2016;55:1092-5.
- [CrossRef] [PubMed] [Google Scholar]
- Metabolic syndrome: Definitions and controversies. BMC Med. 2011;9:48.
- [CrossRef] [PubMed] [Google Scholar]
- Psoriasis and the metabolic syndrome. Clin Dermatol. 2018;36:21-8.
- [CrossRef] [PubMed] [Google Scholar]
- An update on psoriasis and metabolic syndrome: A meta-analysis of observational studies. PLoS One. 2017;12:e0181039.
- [CrossRef] [PubMed] [Google Scholar]
- Relationship between psoriasis and metabolic syndrome: A systematic review. G Ital Dermatol Venereol. 2016;151:663-77.
- [Google Scholar]
- Severe psoriasis-Oral therapy with a new retinoid. Dermatologica. 1978;157:238-44.
- [CrossRef] [PubMed] [Google Scholar]
- Nail psoriasis severity index: A useful tool for evaluation of nail psoriasis. J Am Acad Dermatol. 2003;49:206-12.
- [CrossRef] [Google Scholar]
- Executive summary of the third report of the national cholesterol education program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (Adult treatment panel III) JAMA. 2001;285:2486-97.
- [CrossRef] [PubMed] [Google Scholar]
- Diagnosis and management of the metabolic syndrome: An American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation. 2005;112:2735-52.
- [CrossRef] [PubMed] [Google Scholar]
- Nail involvement in adult patients with plaque-type psoriasis: Prevalence and clinical features. An Bras Dermatol. 2015;90:314-9.
- [CrossRef] [PubMed] [Google Scholar]
- The relationship of obesity with the severity of psoriasis: A systematic review. J Cutan Med Surg. 2015;19:450-6.
- [CrossRef] [PubMed] [Google Scholar]
- Metabolic syndrome in psoriasis among urban South Indians: A case control study using SAM-NCEP criteria. J Clin Diagn Res. 2017;11:WC01-4.
- [CrossRef] [PubMed] [Google Scholar]
- Prevalence of metabolic syndrome in Chinese psoriasis patients: A hospital-based cross-sectional study. J Diabetes Investig. 2018;9:39-43.
- [CrossRef] [PubMed] [Google Scholar]
- Prevalence of metabolic syndrome in patients with psoriasis: A population-based study in the United Kingdom. J Invest Dermatol. 2012;132:556-62.
- [CrossRef] [PubMed] [Google Scholar]
- Clinical features and severity of psoriasis: A comparison of facial and nonfacial involvement in Iran. Arch Iran Med. 2013;16:25-8.
- [Google Scholar]
- A comprehensive review on metabolic syndrome. Cardiol Res Pract. 2014;2014:943162.
- [CrossRef] [PubMed] [Google Scholar]






