Translate this page into:
Fixed drug eruption and generalised erythema following etoricoxib
Correspondence Address:
Mary Augustine
Department of Dermatology, St. John's Medical College Hospital, Sarjapur Road, Bangalore - 560 034, Karnataka
India
| How to cite this article: Augustine M, Sharma P, Stephen J, Jayaseelan E. Fixed drug eruption and generalised erythema following etoricoxib. Indian J Dermatol Venereol Leprol 2006;72:307-309 |
 |
 |
Sir,
Non steroidal anti-inflammatory drugs (NSAIDs) are among the most widely used medications - both by prescription and over the counter. The newer NSAIDs, inhibitors of the cyclo-oxygenase enzyme-2 (COX-2 inhibitors), are fast becoming the drugs of first choice in the treatment of acute pain, chronic pain and most rheumatic conditions. These compounds blunt prostaglandin production through inhibition of cyclooxygenase-2 (COX-2) while sparing cyclooxygenase-1 (COX-1), and have been shown to cause significantly fewer serious gastrointestinal adverse events such as ulceration and bleeding, than the nonselective NSAIDs.[1] Etoricoxib, one of the newer COX-2 inhibitors, has enhanced biochemical COX-2 selectivity over that of the other drugs in this category: rofecoxib and celecoxib.[2] Though, adverse cutaneous effects to celecoxib and rofecoxib have been reported, there has been no report of cutaneous side effects to etoricoxib so far. We report a case of fixed drug eruption and generalized erythema occurring simultaneously following etoricoxib.
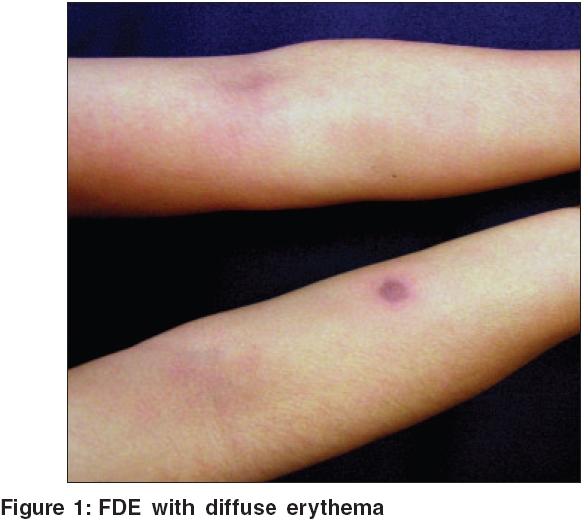
A 38-year-old female, doctor by profession, developed a 1.5 cm size, well circumscribed, round, erythematous patch on the right forearm, 3 days following ingestion of etoricoxib that was prescribed for bursitis of right knee. In the next few days, the center of the patch developed a blister and necrosis which later healed with residual hyperpigmentation. She had taken various NSAIDs many times in the past and rofecoxib on more than two occasions. Celecoxib had been taken at least for one week, once, about two years back. Etoricoxib was taken once, for one week, 5 months back. There were no adverse effects to any of the drugs previously. Therefore the possibility of a drug eruption was not thought of and a diagnosis of ′insect bite reaction′ was considered. Two months after the lesion healed, the patient took a single tablet of etoricoxib again. She noticed erythema, itching and burning over the old lesion within two hours [Figure - 1]. In addition, over the next three to four hours she developed generalized itching and burning sensation followed by intense erythema all over the body. Nikolsky′s sign was negative. There was no mucosal involvement. The patient had neither fever nor other constitutional symptoms. No systemic abnormalities were found on physical and routine laboratory examination. A histopathological examination was not performed as the patient did not consent for skin biopsy.
A drug reaction was diagnosed and systemic steroids were administered. Though most of the symptoms and signs gradually subsided in ten days, mild acral dusky erythema persisted for 4 weeks. The lesion over the right forearm developed a small blister and healed with a larger area of residual pigmentation. The erythema over the rest of the body subsided leaving behind no residual pigmentation.
Since a reliable positive oral re-challenge had already taken place, although inadvertently; further confirmatory tests were not carried out immediately. However, a patch test with etoricoxib 10% in petrolatum was done six months later. Erythema and edema which was double the size of the old patch was seen within eight hours, over the healed FDE lesion, whereas the non-lesional control area did not react.
Fixed drug eruption (FDE) characteristically presents as a round, sharply circumscribed, pruritic or burning, edematous patch with violaceous or dusky erythema.[3] Vesicles or bullae may develop. It heals leaving a hyper-pigmented patch and recurs at the same site on drug rechallenge. The residual pigmentation and recurrence of lesion at the same site are the typical features of FDE. Additional lesions may develop with drug rechallenge. Although a histopathological examination was not performed in our patient, the typical round patch with bulla, the residual pigmentation on healing and the recurrence of the rash at the same site, support a diagnosis of fixed drug eruption. The severity of the patch test reaction confirms etoricoxib as the causative drug.
An unusual feature of this case, however, was the occurrence of two different types of cutaneous adverse reactions simultaneously to the same drug. Clinically the patient had a FDE and a generalized erythematous rash. Although very rare, occurrence of more than one type of cutaneous reactions to the same drug has been reported.[4] Most of the known adverse cutaneous reactions to coxibs have been attributed to either celecoxib or rofecoxib. They include: urticaria/angioedema (by far the most common), Sweet′s syndrome, vasculitis, erythema multiforme, Stevens Johnson syndrome, toxic epidermal necrolysis (TEN) and maculopapular rash.[5],[6],[7] To the best of our knowledge cutaneous reactions to etoricoxib have not been reported so far.
The NSAIDs and coxibs with a sulfonamide structure (celecoxib and valdecoxib) could possibly cross react with sulfonamides.[8] The sulfonamide-type reactions (erythema multiforme, Stevens Johnson syndrome, toxic epidermal necrolysis (TEN) and maculopapular rash) were found to be twice as common with celecoxib as with rofecoxib.[5] The pathogenesis of these reactions is likely to be the same as for sulfonamide induced reactions - T cell mediated type IV hypersensitivity reaction. However, Shapiro et al in their study on the safety of celecoxib in 28 patients with a history of sulfonamide allergy found cross reactivity between celecoxib and sulfonamides to be low.[5]
The coxibs have generally been found to be safe even in patients allergic to the classic NSAIDs. Sanchez-Borges et al , in their review of cutaneous reactions to selective COX-2 inhibitors, reported that, among patients previously exhibiting urticaria or angioedema triggered by classic NSAIDs, only 1.6% developed urticaria or angioedema to rofecoxib and 11.2% to celecoxib.[5] However, in the present case, the patient had been tolerating various NSAIDs in the past but reacted to a coxib.
As the patient had taken rofecoxib on more than two occasions, with no side effects, it appears that there may not necessarily be cross reactivity between different coxibs.
To conclude, cutaneous adverse reactions to coxibs continue to be reported. Although these drugs are considered safer in individuals sensitive to other NSAIDs, this case suggests that the reverse could also be true.
| 1. |
Fitzgerald GA, Patrono C. The coxibs, selective inhibitors of cyclooxygenase-2. N Engl J Med 2001;345:433-42.
[Google Scholar]
|
| 2. |
Cochrane DJ, Jarvis B, Keating GM. Etoricoxib. Drugs 2002;62:2637-51;discussion 2652-3.
[Google Scholar]
|
| 3. |
Korkij W, Soltani K. Fixed drug eruption: A brief review. Arch Dermatol 1984;120:520-4.
[Google Scholar]
|
| 4. |
Gupta PK, Luniya AK, Gupta NK, Tiwari ML. Coexistence of fixed drug eruptions and Stevens Johnson syndrome due to thiacetazone in a patient of pulmonary tuberculosis. Indian J Chest Dis Allied Sci 1983;25:152-4.
[Google Scholar]
|
| 5. |
Sαnchez BM, Capriles HA, Caballero FF. Adverse Reactions to Selective Cyclooxygenase-2 Inhibitors (Coxibs). Am J Ther 2004;11:494-500.
[Google Scholar]
|
| 6. |
Fye KH, Crowley E, Berger TG, Le Boit PE, Connolly MK. Celecoxib-induced Sweet's syndrome. J Am Acad Dermatol 2001;45:300-2.
[Google Scholar]
|
| 7. |
Schneider F, Meziani F, Chartier C, Alt M, Jaeger A. Fatal allergic vasculitis associated with celecoxib. Lancet 2002;359:852-3
[Google Scholar]
|
| 8. |
Sarkar R, Kaur C, Kanwar AJ. Extensive fixed drug eruption to nimesulide with cross-sensitivity to sulfonamides in a child. Pediatr Dermatol 2002;19:553-4.
[Google Scholar]
|
Fulltext Views
4,243
PDF downloads
2,333





