Translate this page into:
Future therapies in melasma: What lies ahead?
Correspondence Address:
Rashmi Sarkar
Department of Dermatology, Maulana Azad Medical College, Bahadur Shah Zafar Marg, Delhi - 110 002
India
| How to cite this article: Sarkar R, Bansal A, Ailawadi P. Future therapies in melasma: What lies ahead?. Indian J Dermatol Venereol Leprol 2020;86:8-17 |
Abstract
Melasma is a common, acquired, symmetrical hypermelanosis. It negatively impacts the patient's quality of life and responds poorly to treatment. Although earlier classified as epidermal and dermal, melasma is now thought to be a complex interaction between epidermal melanocytes, keratinocytes, dermal fibroblasts, mast cells, and vascular endothelial cells. Factors influencing melasma may include inflammation, reactive oxygen species, ultraviolet radiation, genetic factors, and hormones. With a better understanding of the pathogenesis of melasma and the realization that targeting melanin synthesis alone is not very effective, treatments focussing on newly implicated factors have been developed. These include agents targeting hyperactive melanocytes, melanosomal transfer to keratinocytes, defective skin barrier, the mast cells, vasculature, and estrogen receptors as well as drugs with anti-inflammatory and antioxidant activity. Many of these newer agents are botanicals with multimodal mechanisms of action that offer a better safety profile when compared with the conventional drugs. There has also been a focus on oral agents such as tranexamic acid, flutamide, and ascorbic acid. It has been suggested that the “triple therapy of the future” may be a combination of hydroquinone, an antiestrogen and a vascular endothelial growth factor inhibitor, as the “ideal” skin-lightening agent.
Introduction
Melasma is a common, acquired, symmetrical hypermelanosis that presents as light to dark brown macules on the face usually over the forehead and malar areas.[1] Women with Fitzpatrick skin types III–V living in areas of increased ultraviolet (UV) light are frequently affected. Melasma is difficult to treat and negative impacts the quality of life.[2],[3],[4],[5],[6] The most commonly implicated etiological factors include genetic predisposition, exposure to UV radiation (UVR) and hormonal influences.[7]
Over the years the understanding of the pathophysiology of melasma has undergone a paradigm shift. Melasma was earlier classified on the basis of the localization of melanosomes as epidermal, dermal, and mixed.[1] However,in vivo reflectance confocal microscopy has revealed that the distribution of melanophages is heterogeneous, suggesting that all melasma is “mixed” with the dermis often showing solar elastosis and increased vascularity as well.[8],[9] Thus, melasma is now thought to be due to a complex interaction between epidermal melanocytes, keratinocytes, dermal fibroblasts, and vascular endothelial cells, with hormonal and genetic factors and exposure to UVR contributing to the variability, dynamicity and the unyielding nature of this process [Figure - 1].[9]
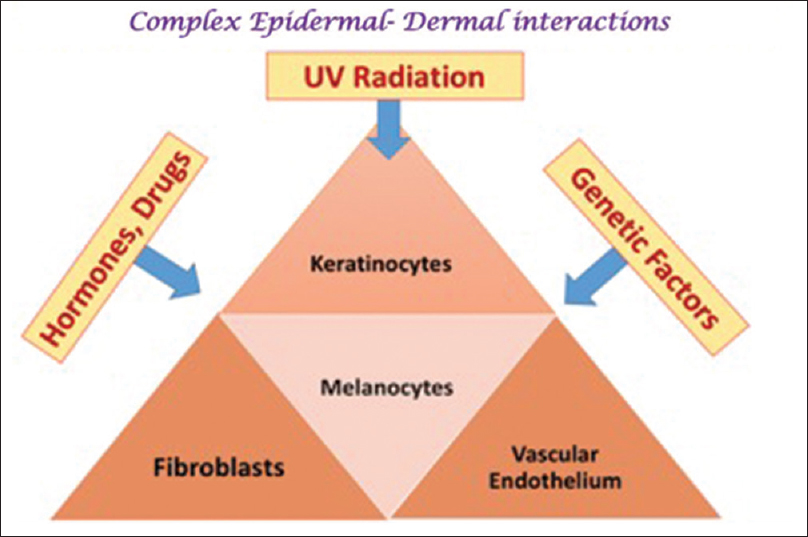 |
| Figure 1 : Melasma – pathogenesis: A complex interaction among epidermal and dermal entities |
The recognition that all melasma is “mixed” has suggested several potential targets for its treatment. Novel agents acting at various levels, from classically targeting the melanogenesis pathway, to acting on hyperactive melanocytes, reducing inflammation and free radical production, and inhibiting melanosomal transfer to keratinocytes have been developed. Some newer drugs may act by restoring the skin barrier or hormonal levels, while others target the dermal vasculature and mast cells.
Melanogenesis and Its Regulation
Melanocytes residing in the skin produce melanin which is then transferred to the adjoining keratinocytes.[10],[11] Melanogenesis is influenced by genetic factors, age, ethnicity, UVR, and drugs.[7] Melanin is synthesized inside melanosomes through a series of steps [Figure - 2] catalyzed by tyrosinase, TYRP1, and TYRP2. The production of these enzymes is controlled by micropthalmia-associated transcription factor (MITF).[12],[13]
 |
| Figure 2: Pathway of melanogenesis and the role of the DHI:DHICA ratio |
The steps of this pathway are:
- Conversion of tyrosine to DOPA; this upregulates tyrosinase activity.[14],[15]
- Tyrosinase then transforms the DOPA to dopaquinone, which then undergoes spontaneous conversion to 5,6 dihydoxyindole (DHI) and dihydroxyindole-2-carboxylic acid (DHICA).[16],[17]
- Both DHI and DHICA are then converted to eumelanin. The activation of TYRP-2 leads to the formation of DHICA-eumelanin associated with a lighter skin phenotype.[17],[18]
- Dopaquinone can also form pheomelanin in the presence of cysteine. A higher eumelanin: pheomelanin ratio contributes to a darker skin phenotype, and TYRP1 and TYRP2 have been found to increase this ratio.[16],[19],[20],[21]
Melanogenesis is regulated by the tyrosine kinase receptor KIT, its ligand SCF (stem cell factor), as well as MITF and melanocortin-1 receptor (MC1R). The activation of MC1R induces a switch from the production of pheomelanin to eumelanin.[22],[23],[24] The SCF-KIT receptor tyrosine kinase pathway activates MITF and regulates melanin production through the induction of tyrosinase.[22]
Most conventional skin-lightening agents are inhibit melanogenesis and its regulation. However, newer therapies targeting other aspects of hyperpigmentation are now being developed.
Hyperactive Melanocytes
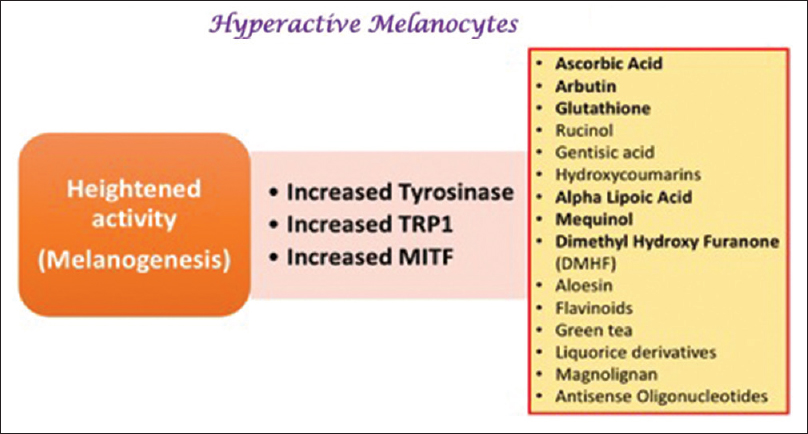
The genes involved in melanogenesis (tyrosinase, TYRP1, TYRP2, and MITF) are upregulated in the lesional skin of patients with melasma, and lesional melanocytes show an increased number of dendrites, mitochondria, golgi bodies, and rough endoplasmic reticulum. These findings suggest that heightened biological activity (rather than an increased number of cells) is responsible for the hyperpigmentation in melasma.[25],[26] Thus, inhibiting the activity of melanocytes in addition to reducing melanin synthesis may be more effective in improving melasma.
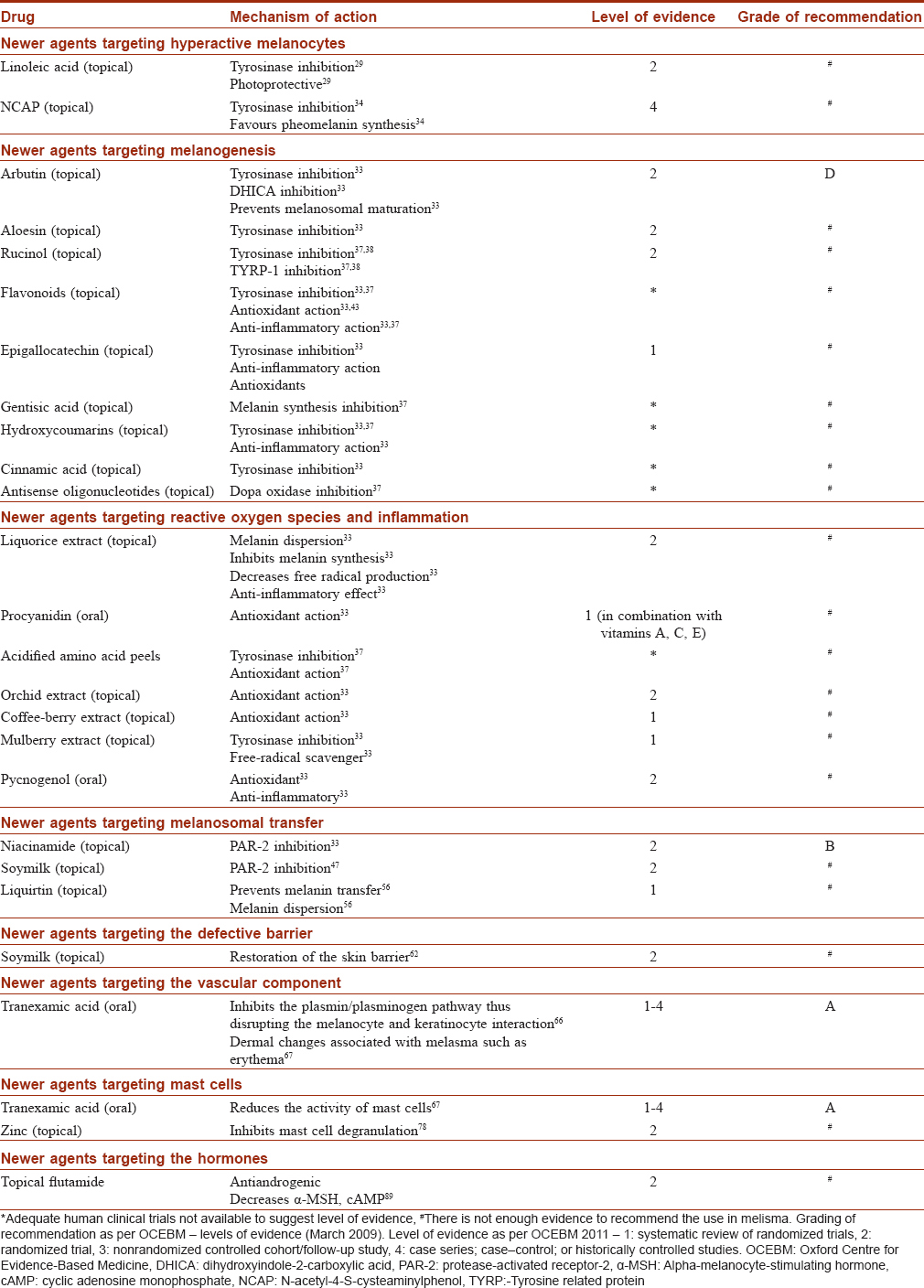
Newer agents targeting melanogenesis and hyperactive melanocytes
Conventional skin-lightening agents such as hydroquinone (HQ) and azelaic acid exert their effects selectively in hyperactive melanocytes i.e. cells with upregulated tyrosinase activity.[27],[28]
Newer agents specifically targeting hyperactive melanocytes
Linoleic acid (topical)
Linoleic acid selectively targets tyrosinase in hyperactive melanocytes and decreases UVB-induced pigmentation.[29] In a 6 week double-blind, randomized controlled trial (RCT), a combination linoleic acid with lincomycin and betamethasone valerate showed greater improvement than a combination of the latter two or the vehicle alone.[30]
Ascorbic acid (topical)
Ascorbic acid decreases the oxidation of dopaquinone and DHICA.[31] In addition, it decreases tyrosinase activity, reduces dermal damage, promotes collagen synthesis and has an antioxidant and photoprotective effects, thus reducing hyperpigmentation. 32 In a 16 week split-face study comparing 5% ascorbic acid (AsA) and 4% HQ cream improvement on the HQ side was seen in 93% of 16 women as compared to 62.5% on the AsA side (P < 0.05). Side effects (SEs) were less frequent in AsA treated patients (6.2% vs 68.7%).[32]
N-acetyl-4-S-cysteaminylphenol (topical)
N-acetyl-4-S-cysteaminylphenol (NCAP) is effective in the treatment of hyperpigmentation[33] It is less irritant and more stable than HQ.[33],[34] It inhibits tyrosinase in hyperactive melanocytes, interferes with the thiol system decreasing intracellular glutathione, and favors pheomelanin synthesis. 34 NCAP 4% produced significant improvement in 66% of 12 patients with melasma with complete resolution in 8%.[33],[34]
Newer agents targeting melanogenesis
Arbutin and deoxyarbutin (topical)
Arbutin is a derivative of HQ. It inhibits tyrosinase and DHICA, and prevents melanosomal maturation. Its effect is dose-dependent and it has fewer SEs when compared with HQ.[33],[34] In an 8 week study of 54 patients with melasma 2.51% arbutin was significantly more effective than placebo in improving melasma.[35] Deoxyarbutin, a synthetic derivative of arbutin, is also effective and safe.[33]
Aloesin (topical)
Aloesin is extracted from aloe vera. It competitively inhibits the conversion of tyrosine to DOPA and DOPA to dopachrome.[33] A dose-dependent skin-lightening effect was noted when aloesin was applied four times daily for 15 days on UV-irradiated human forearm skin.[36]
Rucinol (topical)
Rucinol (4-n-butylresorcinol) is a phenol derivative that inhibits tyrosinase and TYRP-1[37],[38] In a randomized, double-blind, vehicle-controlled study 4-n-butylresorcinol 0.1% cream was shown to be effective in 20 patients with melasma.[38]
Flavonoids (topical)
Flavonoids are benzopyrene derivatives; they have anti-inflammatory and antioxidant properties and are competitive inhibitors of tyrosinase.[33],[37] Hesperidin is a flavonoid that protects against UVR-induced free radical damage.[39]
Epigallocatechin gallate and ellagic acid (topical)
Epigallocatechin gallate is a phenolic compound extracted from green tea.[33],[37] It inhibits melanogenesis and also has significant anti-inflammatory, antioxidant, and anticancer properties.[33] Ellagic acid is a polyphenol derivative found in green tea, strawberries, and pomegranate. It can inhibit tyrosinase and melanocyte proliferation.[40]
Gentisic acid (topical)
Gentisic acid is a compound extracted from gentian roots. It inhibits melanin synthesis.[37],[41]
Hydroxycoumarins (topical)
Hydroxycoumarins are naturally occurring lactones that inhibit tyrosinase and also have antioxidant activity.[37] Umbelliferone (7-hydroxycoumarin) has, in addition, anti-inflammatory action.[33]
Cinnamic acid (topical)
Cinnamic acid is derived from ginseng. It inhibits tyrosinase[33],[42] and is more potent than HQ.[43]
Antisense oligonucleotides (topical)
Antisense oligonucleotides act as skin-lightening agents by downregulating the production of enzymes involved in melanogenesis and decreasing the activity of DOPA oxidase.[37],[44]
Role of Inflammation and Reactive Oxygen Species
Reactive oxygen species (ROS) may be produced by several environmental factors including UVR. ROS can cause oxidative damage to the skin by interacting with cellular lipids, proteins, DNA, and carbohydrates.[45],[46] Excess ROS can activate tyrosinase and increase melanin synthesis and melasma is associated with a disruption of the oxidant–antioxidant balance.[33],[47] Several interleukins and cytokines can stimulate melanocyte proliferation, upregulate melanin production, and enhance melanosome transfer.[48],[49] Thus, drugs with an antioxidant and anti-inflammatory action are being investigated for their potential as therapeutic agents in melasma [Figure - 3].[50]
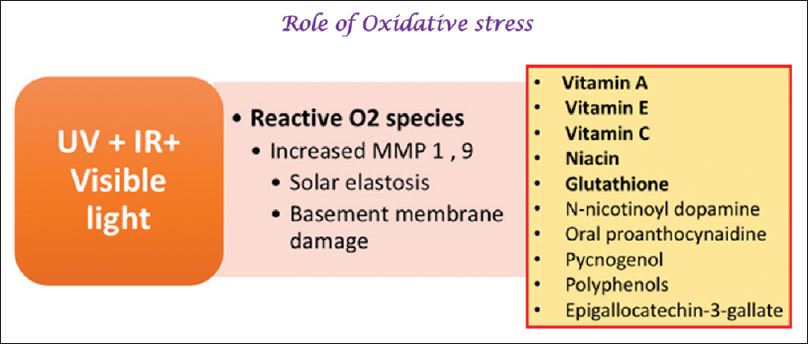 |
| Figure 3: Role of drugs with antioxidant and anti-inflammatory activity |
Newer agents targeting ROS and inflammation
Liquorice extract (topical)
Liquorice extract derived from the root of Glycyrrhiza glabra inhibits melanin synthesis, causes melanin dispersion, and decreases ROS production.[33],[37] It also has anti-inflammatory effects and can decrease UVB-induced hyperpigmentation in guinea pigs when used topically for 3 weeks.[51]
Proanthocyanidin (oral)
Proanthocyanidin extracted from grape seeds has significant antioxidant action and has been shown to be beneficial in melasma in several studies.[52],[53] In a study of women with melasma, proanthocyanidin administered orally for 6 months resulted in significant skin-lightening in 10 of the 12 women (83%, P < 0.01).[53]
Acidified amino acid peels (topical)
Topical acidified amino acid peels with a pH similar to that of skin have significant antioxidant and tyrosinase inhibitory action and have fewer SEs as compared with glycolic acid.[37],[54]
Orchid extract (topical)
Orchid extracts possess strong antioxidant activity. Orchid extract was as effective as 3% vitamin C in a study of 48 patients with melasma.[33],[37]
Coffeeberry extract (topical)
Coffeeberry extract has antioxidant properties and it was found to significantly reduce hyperpigmentation and photodamage in a 6-week study of 40 patients with melasma.[33],[37]
Mulberry extract (topical)
Derived from the plant Mores alba, mulberry extract is a free radical scavenger and inhibits tyrosinase.[33],[47] The concentration producing 50% inhibition of tyrosinase activity is lower for mulberry extract than HQ and it markedly improved the Melasma Area and Severity Index (MASI) score in an RCT in 50 patients with melasma.[47]
Pycnogenol (oral)
Derived from the bark of Pinus pinaster, this agent has significant antioxidant and anti-inflammatory properties, and an oral formulation has been found to be beneficial in melasma.[33],[55]
Other agents
Polypodium leucomatous extracts act by inhibition of UV induced ROS generation, including superoxide anions. AsA and alpha tocopherol are strong anti-inflammatory agents with a marked antioxidant mechanism.[33]
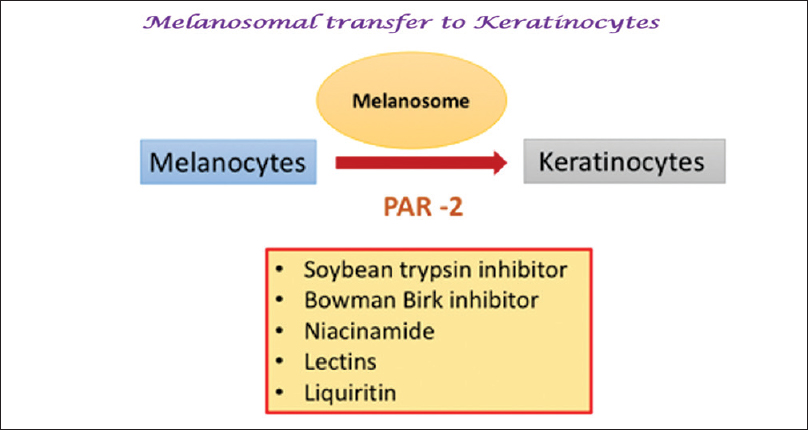
Melanosomal Transfer: Protease-Activated Receptor 2
Melanosomes are transferred from epidermal melanocytes to neighboring keratinocytes as part of the epidermal-melanin unit.[48] Drugs inhibiting the keratinocyte protease-activated receptor 2 (PAR-2) inhibit melanosomal transfer and have been shown to be effective in melasma.[33],[48]
Newer agents targeting melanosomal transfer
Niacinamide (topical)
Niacinamide or vitamin B3, the active amide of niacin, interferes with melanosomal transfer to the surrounding keratinocytes by inhibiting PAR-2.[33],[46]
Liquirtin (topical)
Liquirtin leads to a skin-lightening effect through dispersion of melanin. A 20% liquiritin cream was shown to be effective in melasma.[56]
Soymilk, soybean (topical)
Serine protease inhibitors (soy trypsin inhibitor (STI) and Bowman–Birk inhibitor (BBI)) found in soybeans have been shown to inhibit melanosome phagocytosis by keratinocytes via inhibition of PAR-2.[48],[57] In a double-blind, study of a soy-containing moisturizer with a broad-spectrum sunscreen in 68 patients, significant improvements in fine wrinkles and pigmentary changes were demonstrated after 3 months of usage.[57]
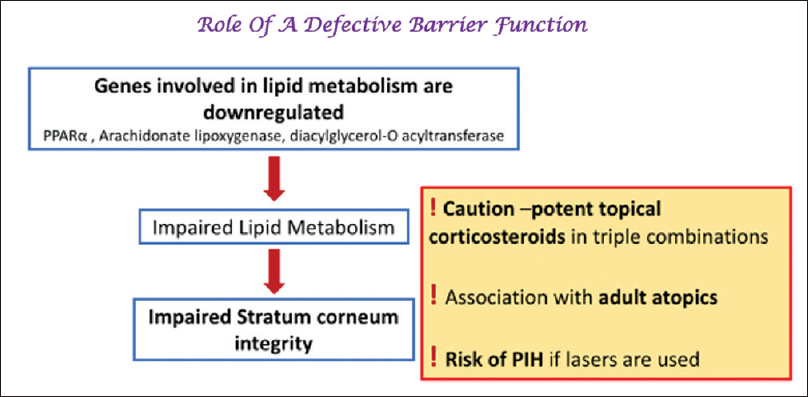
The Defective Skin Barrier in Melasma
Impaired stratum corneum integrity has been demonstrated in melasma. A UVR-induced, as well well as a de novo downregulation of several lipid metabolism genes (such as peroxisome proliferator-activated receptor alpha) results in impaired production of free fatty acids leading to a disrupted epidermal barrier [Figure - 4].[58],[59],[60],[61],[62]
 |
| Figure 4: Role of a defective skin barrier in melisma |
Newer agents targeting the defective barrier
Soy (topical)
Topically applied, active soy moisturizer containing nondenatured serine protease inhibitors (STI and BBI) can decrease UVB-induced pigmentation by restoring the skin barrier.[57]
The Vascular Component
Increased synthesis of proangiogenic factors such as vascular endothelial growth factor (VEGF) results in the proliferation of the dermal vessels. VEGF may increase melanin synthesis through VEGF receptors located on melanocytes.[63],[64] UVR-induced release of plasminogen from dermal vessels may also enhance melanogenesis.[65]
Newer agents targeting the vascular component
Tranexamic acid (oral)
Tranexamic acid (TXA) inhibits the plasmin/plasminogen pathway. This results in interference in melanocyte and keratinocyte interactions thus inhibiting melanin synthesis [Figure - 5].[66] TXA also influences several other dermal changes associated with melasma such as erythema and reduces both epidermal and dermal pigmentation.[67] Several studies with oral TXA have demonstrated response rates of up to 89.7%, with visible lightening observed at around 2 months.[68],[69],[70],[71] Padhi et al., observed faster improvement in melasma when a fluocinolone-based triple combination cream was used along with TXA.[72]
 |
| Figure 5: Role of vascular endothelium and mast cells – tranexamic acid inhibits the plasminogen pathway and decreases the activity of mast cells. Zinc primarily affects mast cell degranulation |
Role of Histamine and the Mast Cells
An increased number of mast cells has been noted in the lesional skin in melasma.[73] UVR induces histamine synthesis and this in turn stimulates proliferation of melanocytes through the H2 receptors. UVR also causes mast cell tryptase activation leading to extracellular matrix degradation and basement membrane disruption.[74],[75] Mast cells can produce VEGF, transforming growth factor-beta (TGF-β), and fibroblast growth factor-2 all of which promote vascular proliferation thus contributing significantly to melasma.[76],[77]
Despite the significant role of mast cells and histamine in the pathogenesis of melasma, antihistamines have failed to demonstrate a significant benefit in the management of melasma.
Newer agents targeting mast cells
Tranexamic acid (oral)
TXA been shown to reduce the activity of mast cells. In a study of 25 women with melasma, oral TXA tablets were administered three times daily and a topical TXA agent was applied twice daily for 8 weeks. On histopathological analysis, mast cells were found to be decreased after treatment suggesting that the effect on mast cells may underly the therapeutic effect of TXA in melasma.[66]
Zinc (topical)
Zinc reduces histamine secretion through inhibition of mast cell degranulation and is also an antioxidant. A significant reduction in melasma was seen in 14 patients using 10% topical zinc sulfate after 3 months of therapy.[78],[79]
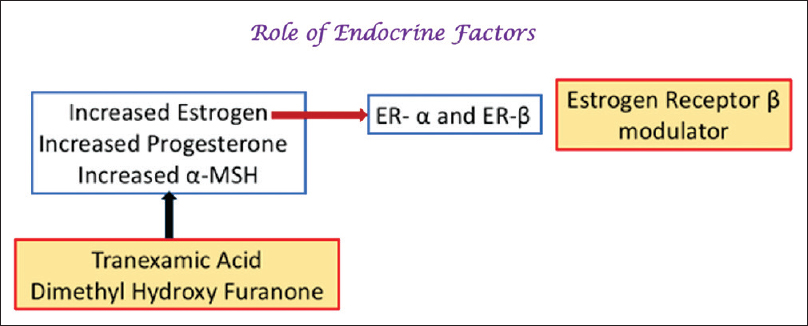
Role of the Estrogen Receptor
Melasma is commonly seen among women in the reproductive age group especially during pregnancy or with oral contraceptive use.[80],[81] Estrogens upregulate the synthesis of enzymes involved in melanin production such as tyrosinase, TRP-1, TRP-2, and MITF, and also upregulate estrogen receptors in the lesional skin.[82],[83],[84],[85],[86]
Thus, drugs inhibiting the effect of estrogen such as selective estrogen receptor modulators (eg., tamoxifen, raloxifene) or aromatase inhibitors (eg., anastrozole, letrozole or exemestane) may be efficacious in the treatment of melasma.[84],[85] Furthermore, this research suggests that the “triple therapy of the future” could include a HQ, an antiestrogen, and a VEGF inhibitor.[87]
Newer agents targeting hormones
Topical flutamide
Flutamide is an antiandrogenic agent that can influence alpha-melanocyte-stimulating hormone and cyclic adenosine monophosphate which are key regulators of melanogenesis and in a randomized trial in 74 women with melasma both 4% HQ cream or 1% flutamide cream were found to equally effective in treating melasma.[88]
Newer Agents With Unique Mechanisms: Potential Targets of the Future
Curcumin (topical)
Curcumin is a bioactive compound extracted from the rhizome of Curcuma longa and its use is well established in traditional Chinese medicine for the treatment of various skin diseases.[89],[90],[91] It inhibits UVB-induced production of ROS and expression of matrix metalloproteinasein vitro by blocking the activation of the UVB-induced mitogen-activated protein kinase, nuclear factor-κB and AP-1 transcription factor signal pathways.[92] Curcumin gel has been found to be useful in the repair of photodamaged skin and the associated pigmentary changes and solar elastosis.[93]
In view of its anti-inflammatory, free radical scavenging, UV-protective activities, curcumin in may serve as a novel skin-lightening agent of the future, both as a topical and an oral preparation.
Lignin peroxidase (topical)
Lignin peroxidase (LP), an enzyme derived from the fungus Phanerochaete chrysosporium. Since lignin is structurally similar to melanin, lignin-degrading enzymes can be utilized to decolorize melanin.[94],[95] Lignin peroxidase is marketed as a formulation containing the active enzyme component and its activator (hydrogen peroxide) which causes the destruction of eumelanin. In 51 Asian patients, LP was found to be more efficacious than HQ 2% with significant results seen as early as 7 days.[96]
Platelet-rich plasma
TGF-β1 released from α-granules in platelets has been shown to cause significant inhibition of melanin synthesis through delayed extracellular signal-regulated kinase activation.[97] PRP therapy may also cause improvement in melasma by releasing platelet-derived growth factor, which causes an increase in skin volume as a result of angiogenesis and synthesis of collagen.[98] A greater than 80% reduction of melasma was seen in a 27-year-old woman treated with three sessions of PRP with no recurrence during follow-up.[99]
Microneedling
Microneedling is commonly used for enhancing the drug delivery of depigmenting agents in melasma. Microneedling under topical anesthesia (two sessions, 1 month apart) along with triple-combination cream applied at night was effective in reducing pigmentation in 22 patients with recalcitrant facial melasma.[100]
Newer sunscreens
Visible light (VL) and infrared light (IR) have been shown to play an important role in hyperpigmentation, especially in the darker skin types (III, IV, or V). VL may induce the production of ROS leading to DNA damage. IR light provokes the activation of the endothelin receptor B and the mitogen-activated protein kinase which facilitate melanogenesis. Sunscreens containing iron-oxide are effective against hyperpigmentation induced by VL. Other novel UV-VL sunscreens that allow absorption of the radiation in the VL spectrum, and systemic antioxidants such as vitamin A, C, and E, carotenoids and beta-carotene may provide additive protection. Nonorganic and organic filters that absorb or reflect IR are currently available and topical antioxidants may be able to offer some protection against IR-related damage. However, their clinical efficacy still remains to be determined.[101],[102]
Conclusion
With improved understanding of the pathogenesis of melasma, novel therapeutic targets with the potential for development of newer therapies have now become available.
Melasma is no longer thought to be a static process but a complex epidermal–dermal dynamic interaction with various cell types, inflammation, oxidative stress, and photodamage all contributing significantly to this process. [Figure - 6]. This implies that the earlier approach of targeting epidermal melanin alone is insufficient and newer drug classes that target other aspects of the pathogenesis of melasma such as hyperactive pendulous melanocytes, inflammation, free radicals, melanosomal transfer, dermal vasculature, hormone receptors, and the defective skin barrier may be effective [Table - 1]and [Table - 1]2.
 |
| Figure 6: Summary of the various new drugs and their mechanism of action. 1–7 represent the mechanisms of development of melasma: 1 – hyperactive melanocytes and melanogenesis; 2 – melanosomal transfer to keratinocytes; 3 – inflammation and reactive oxygen species; 4 – skin barrier; 5 – dermal vasculature; 6 – mast cells and histamine; 7 – estrogen receptors. ER: estrogen receptors, NCAP: N-acetyl-4-S-cysteaminylphenol, TXA: tranexamic acid |
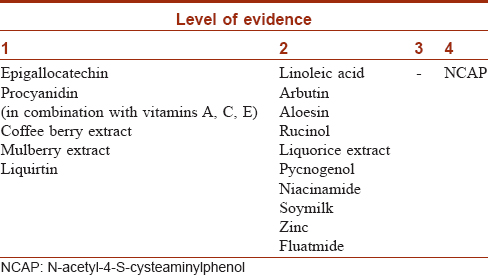
There are few studies of this ever-expanding list of drugs for melasma and it is imperative to investigate the efficacy and safety profile of these drugs in large scale trials. Despite encouraging results in limited studies most have been inferior to HQ and it is premature to recommend them as absolute alternatives to conventional drugs. It is likely that these newer drugs may play a role only as add-on or second-line drugs or for as maintenance therapy.
Acknowledgement
Jaypee Brothers Publishers: for Figures 1–5: Reproduced with permission from Bansal A, Ailawadi P, Sarkar R. Treatment of melasma. In: Sarkar R, editor. Melasma: A Monograph. 2nd ed. India: Jaypee Brothers Publishers; 2018.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
| 1. |
Sanchez NP, Pathak MA, Sato S, Fitzpatrick TB, Sanchez JL, Mihm MC Jr. Melasma: A clinical, light microscopic, ultrastructural, and immunofluorescence study. J Am Acad Dermatol 1981;4:698-710.
[Google Scholar]
|
| 2. |
Balkrishnan R, McMichael AJ, Camacho FT, Saltzberg F, Housman TS, Grummer S, et al. Development and validation of a health-related quality of life instrument for women with melasma. Br J Dermatol 2003;149:572-7.
[Google Scholar]
|
| 3. |
Cestari TF, Balkrishnan R, Weber MB, Prati C, Menegon DB, Mazzotti NG, et al. Translation and cultural adaptation to Portuguese of a quality of life questionnaire for patients with melasma. Med Cutan Iber Lat Am 2006;34:270-4.
[Google Scholar]
|
| 4. |
Cestari TF, Hexsel D, Viegas ML, Azulay L, Hassun K, Almeida AR, et al. Validation of a melasma quality of life questionnaire for Brazilian Portuguese language: The melasQoL-BP study and improvement of qoL of melasma patients after triple combination therapy. Br J Dermatol 2006;156 Suppl 1:13-20.
[Google Scholar]
|
| 5. |
Freitag FM, Cestari TF, Leopoldo LR, Paludo P, Boza JC. Effect of melasma on quality of life in a sample of women living in Southern Brazil. J Eur Acad Dermatol Venereol 2008;22:655-62.
[Google Scholar]
|
| 6. |
Dominguez AR, Balkrishnan R, Ellzey AR, Pandya AG. Melasma in Latina patients: Cross-cultural adaptation and validation of a quality-of-life questionnaire in Spanish language. J Am Acad Dermatol 2006;55:59-66.
[Google Scholar]
|
| 7. |
Grimes PE. Melasma. Etiologic and therapeutic considerations. Arch Dermatol 1995;131:1453-7.
[Google Scholar]
|
| 8. |
Kang HY, Ortonne JP. What should be considered in treatment of melasma. Ann Dermatol 2010;22:373-8.
[Google Scholar]
|
| 9. |
Kwon SH, Hwang YJ, Lee SK, Park KC. Heterogeneous pathology of melasma and its clinical implications. Int J Mol Sci 2016;17. pii: E824.
[Google Scholar]
|
| 10. |
Lin JY, Fisher DE. Melanocyte biology and skin pigmentation. Nature 2007;445:843-50.
[Google Scholar]
|
| 11. |
Delevoye C. Melanin transfer: The keratinocytes are more than gluttons. J Invest Dermatol 2014;134:877-9.
[Google Scholar]
|
| 12. |
Schiaffino MV. Signaling pathways in melanosome biogenesis and pathology. Int J Biochem Cell Biol 2010;42:1094-104.
[Google Scholar]
|
| 13. |
Marks MS, Seabra MC. The melanosome: Membrane dynamics in black and white. Nat Rev Mol Cell Biol 2001;2:738-48.
[Google Scholar]
|
| 14. |
Slominski A, Moellmann G, Kuklinska E. L-tyrosine, L-dopa, and tyrosinase as positive regulators of the subcellular apparatus of melanogenesis in bomirski Ab amelanotic melanoma cells. Pigment Cell Res 1989;2:109-16.
[Google Scholar]
|
| 15. |
Slominski A, Zmijewski MA, Pawelek J. L-tyrosine and L-dihydroxyphenylalanine as hormone-like regulators of melanocyte functions. Pigment Cell Melanoma Res 2012;25:14-27.
[Google Scholar]
|
| 16. |
Bolognia JL, Pawelek JM. Biology of hypopigmentation. J Am Acad Dermatol 1988;19:217-55.
[Google Scholar]
|
| 17. |
Taieb A, Cario-Andre M, Brigand S, Picardo M. Inhibitors and enhancers of melanogenesis. In: Riley PA, Borovansky J, editors. Melanins and Melanosomes: Biosynthesis, Biogenesis, Physiological, and Pathological Functions. 1st ed. Wiley Blackwell; Weinheim, Germany; 2011. p. 117-54.
[Google Scholar]
|
| 18. |
Palumbo A, Solano F, Misuraca G, Aroca P, Garcia Borron JC, Lozano JA, et al. Comparative action of dopachrome tautomerase and metal ions on the rearrangement of dopachrome. Biochim Biophys Acta 1991;1115:1-5.
[Google Scholar]
|
| 19. |
Gupta S. Skin colour: No hiding in the dark. Nature 2014;515:S121-3.
[Google Scholar]
|
| 20. |
Wu S, Han J, Laden F, Qureshi AA. Long-term ultraviolet flux, other potential risk factors, and skin cancer risk: A cohort study. Cancer Epidemiol Biomarkers Prev 2014;23:1080-9.
[Google Scholar]
|
| 21. |
Videira IF, Moura DF, Magina S. Mechanisms regulating melanogenesis. An Bras Dermatol 2013;88:76-83.
[Google Scholar]
|
| 22. |
Hou L, Panthier JJ, Arnheiter H. Signaling and transcriptional regulation in the neural crest-derived melanocyte lineage: Interactions between KIT and MITF. Development 2000;127:5379-89.
[Google Scholar]
|
| 23. |
Slominski A, Tobin DJ, Shibahara S, Wortsman J. Melanin pigmentation in mammalian skin and its hormonal regulation. Physiol Rev 2004;84:1155-228.
[Google Scholar]
|
| 24. |
Hida T, Wakamatsu K, Sviderskaya EV, Donkin AJ, Montoliu L, Lynn Lamoreux M, et al. Agouti protein, mahogunin, and attractin in pheomelanogenesis and melanoblast-like alteration of melanocytes: A cAMP-independent pathway. Pigment Cell Melanoma Res 2009;22:623-34.
[Google Scholar]
|
| 25. |
Grimes PE, Yamada N, Bhawan J. Light microscopic, immunohistochemical, and ultrastructural alterations in patients with melasma. Am J Dermatopathol 2005;27:96-101.
[Google Scholar]
|
| 26. |
Kang WH, Yoon KH, Lee ES, Kim J, Lee KB, Yim H, et al. Melasma: Histopathological characteristics in 56 Korean patients. Br J Dermatol 2002;146:228-37.
[Google Scholar]
|
| 27. |
Smith CJ, O'Hare KB, Allen JC. Selective cytotoxicity of hydroquinone for melanocyte-derived cells is mediated by tyrosinase activity but independent of melanin content. Pigment Cell Res 1988;1:386-9.
[Google Scholar]
|
| 28. |
Nazzaro-Porro M. Azelaic acid. J Am Acad Dermatol 1987;17:1033-41.
[Google Scholar]
|
| 29. |
Ando H, Ryu A, Hashimoto A, Oka M, Ichihashi M. Linoleic acid and alpha-linolenic acid lightens ultraviolet-induced hyperpigmentation of the skin. Arch Dermatol Res 1998;290:375-81.
[Google Scholar]
|
| 30. |
Lee MH, Kim HJ, Ha DJ, Paik JH, Kim HY. Therapeutic effect of topical application of linoleic acid and lincomycin in combination with betamethasone valerate in melasma patients. J Korean Med Sci 2002;17:518-23.
[Google Scholar]
|
| 31. |
Ros JR, Rodríguez-López JN, García-Cánovas F. Effect of L-ascorbic acid on the monophenolase activity of tyrosinase. Biochem J 1993;295(Pt 1):309-12.
[Google Scholar]
|
| 32. |
Espinal-Perez LE, Moncada B, Castanedo-Cazares JP. A double-blind randomized trial of 5% ascorbic acid vs. 4% hydroquinone in melasma. Int J Dermatol 2004;43:604-7.
[Google Scholar]
|
| 33. |
Sarkar R, Arora P, Garg KV. Cosmeceuticals for hyperpigmentation: What is available? J Cutan Aesthet Surg 2013;6:4-11.
[Google Scholar]
|
| 34. |
Picardo M, Carrera M. New and experimental treatments of cloasma and other hypermelanoses. Dermatol Clin 2007;25:353-62, ix.
[Google Scholar]
|
| 35. |
Morag M, Nawrot J, Siatkowski I, Adamski Z, Fedorowicz T, Dawid-Pac R, et al. Adouble-blind, placebo-controlled randomized trial of serratulae quinquefoliae folium, a new source of β-arbutin, in selected skin hyperpigmentations. J Cosmet Dermatol 2015;14:185-90.
[Google Scholar]
|
| 36. |
Choi S, Lee SK, Kim JE, Chung MH, Park YI. Aloesin inhibits hyperpigmentation induced by UV radiation. Clin Exp Dermatol 2002;27:513-5.
[Google Scholar]
|
| 37. |
Sarkar R, Chugh S, Garg VK. Newer and upcoming therapies for melasma. Indian J Dermatol Venereol Leprol 2012;78:417-28.
[Google Scholar]
|
| 38. |
Huh SY, Shin JW, Na JI, Huh CH, Youn SW, Park KC. The efficacy and safety of 4-n-butylresorcinol 0.1% cream for the treatment of melasma: A randomized controlled split-face trial. Ann Dermatol 2010;22:21-5.
[Google Scholar]
|
| 39. |
Proteggente AR, Basu-Modak S, Kuhnle G, Gordon MJ, Youdim K, Tyrrell R, et al. Hesperetin glucuronide, a photoprotective agent arising from flavonoid metabolism in human skin fibroblasts. Photochem Photobiol 2003;78:256-61.
[Google Scholar]
|
| 40. |
Yoshimura M, Watanabe Y, Kasai K, Yamakoshi J, Koga T. Inhibitory effect of an ellagic acid-rich pomegranate extract on tyrosinase activity and ultraviolet-induced pigmentation. Biosci Biotechnol Biochem 2005;69:2368-73.
[Google Scholar]
|
| 41. |
Curto EV, Kwong C, Hemersdorfer H, Glatt H, Santis C, Virador V, et al. Inhibitors of mammalian melanocyte tyrosinase: In vitro comparisons of alkyl esters of gentisic acid with other putative inhibitors. Biochem Pharmacol 1999;57:663-72.
[Google Scholar]
|
| 42. |
Takahashi T, Miyazawa M. Tyrosinase inhibitory activities of cinnamic acid analogues. Pharmazie 2010;65:913-8.
[Google Scholar]
|
| 43. |
Tan C, Zhu W, Lu Y. Aloin, cinnamic acid and sophorcarpidine are potent inhibitors of tyrosinase. Chin Med J (Engl) 2002;115:1859-62.
[Google Scholar]
|
| 44. |
Lazou K, Sadick NS, Kurfurst R, Bonnet Duquennoy M, Neveu M, Nizard C, et al. The use of antisense strategy to modulate human melanogenesis. J Drugs Dermatol 2007;6:s2-7.
[Google Scholar]
|
| 45. |
Maeda K, Hatao M. Involvement of photooxidation of melanogenic precursors in prolonged pigmentation induced by ultraviolet A. J Invest Dermatol 2004;122:503-9.
[Google Scholar]
|
| 46. |
Babior BM. Phagocytes and oxidative stress. Am J Med 2000;109:33-44.
[Google Scholar]
|
| 47. |
Lee SH, Choi SY, Kim H, Hwang JS, Lee BG, Gao JJ, et al. Mulberroside F isolated from the leaves of Morus alba inhibits melanin biosynthesis. Biol Pharm Bull 2002;25:1045-8.
[Google Scholar]
|
| 48. |
Paine C, Sharlow E, Liebel F, Eisinger M, Shapiro S, Seiberg M, et al. An alternative approach to depigmentation by soybean extracts via inhibition of the PAR-2 pathway. J Invest Dermatol 2001;116:587-95.
[Google Scholar]
|
| 49. |
Smit N, Vicanova J, Pavel S. The hunt for natural skin whitening agents. Int J Mol Sci 2009;10:5326-49.
[Google Scholar]
|
| 50. |
Grimes PE. An efficacy study of 3 commercially available hydroquinone 4% treatments for melasma. Cutis 2007;80:497-502.
[Google Scholar]
|
| 51. |
Yokota T, Nishio H, Kubota Y, Mizoguchi M. The inhibitory effect of glabridin from licorice extracts on melanogenesis and inflammation. Pigment Cell Res 1998;11:355-61.
[Google Scholar]
|
| 52. |
Handog EB, Galang DA, de Leon-Godinez MA, Chan GP. A randomized, double-blind, placebo-controlled trial of oral procyanidin with Vitamins A, C, E for melasma among filipino women. Int J Dermatol 2009;48:896-901.
[Google Scholar]
|
| 53. |
Yamakoshi J, Sano A, Tokutake S, Saito M, Kikuchi M, Kubota Y, et al. Oral intake of proanthocyanidin-rich extract from grape seeds improves chloasma. Phytother Res 2004;18:895-9.
[Google Scholar]
|
| 54. |
Ilknur T, Biçak MU, Demirtaşoǧlu M, Ozkan S. Glycolic acid peels versus amino fruit acid peels in the treatment of melasma. Dermatol Surg 2010;36:490-5.
[Google Scholar]
|
| 55. |
Ni Z, Mu Y, Gulati O. Treatment of melasma with pycnogenol. Phytother Res 2002;16:567-71.
[Google Scholar]
|
| 56. |
Amer M, Metwalli M. Topical liquiritin improves melasma. Int J Dermatol 2000;39:299-301.
[Google Scholar]
|
| 57. |
Wallo W, Nebus J, Leyden JJ. Efficacy of a soy moisturizer in photoaging: A double-blind, vehicle-controlled, 12-week study. J Drugs Dermatol 2007;6:917-22.
[Google Scholar]
|
| 58. |
Lee DJ, Lee J, Ha J, Park KC, Ortonne JP, Kang HY. Defective barrier function in melasma skin. J Eur Acad Dermatol Venereol 2012;26:1533-7.
[Google Scholar]
|
| 59. |
Elias PM, Menon G, Wetzel BK, Williams JJ. Evidence that stress to the epidermal barrier influenced the development of pigmentation in humans. Pigment Cell Melanoma Res 2009;22:420-34.
[Google Scholar]
|
| 60. |
Kang HY, Suzuki I, Lee DJ, Ha J, Reiniche P, Aubert J, et al. Transcriptional profiling shows altered expression of Wnt pathway- and lipid metabolism-related genes as well as melanogenesis-related genes in melasma. J Invest Dermatol 2011;131:1692-700.
[Google Scholar]
|
| 61. |
Kim EJ, Jin XJ, Kim YK, Oh IK, Kim JE, Park CH, et al. UV decreases the synthesis of free fatty acids and triglycerides in the epidermis of human skin in vivo, contributing to development of skin photoaging. J Dermatol Sci 2010;57:19-26.
[Google Scholar]
|
| 62. |
Mao-Qiang M, Fowler AJ, Schmuth M, Lau P, Chang S, Brown BE, et al. Peroxisome-proliferator-activated receptor (PPAR)-gamma activation stimulates keratinocyte differentiation. J Invest Dermatol 2004;123:305-12.
[Google Scholar]
|
| 63. |
Kim EH, Kim YC, Lee ES, Kang HY. The vascular characteristics of melasma. J Dermatol Sci 2007;46:111-6.
[Google Scholar]
|
| 64. |
Kim EJ, Park HY, Yaar M, Gilchrest BA. Modulation of vascular endothelial growth factor receptors in melanocytes. Exp Dermatol 2005;14:625-33.
[Google Scholar]
|
| 65. |
Morelli JG, Norris DA. Influence of inflammatory mediators and cytokines on human melanocyte function. J Invest Dermatol 1993;100:191S-5S.
[Google Scholar]
|
| 66. |
Maeda K, Tomitab Y. Mechanism of the inhibitory effect of tranexamic acid on melanogenesis in cultured human melanocytes in the presence of keratinocyte-conditioned medium. J Health Sci 2007;53:389-96.
[Google Scholar]
|
| 67. |
Na JI, Choi SY, Yang SH, Choi HR, Kang HY, Park KC, et al. Effect of tranexamic acid on melasma: A clinical trial with histological evaluation. J Eur Acad Dermatol Venereol 2013;27:1035-9.
[Google Scholar]
|
| 68. |
Tse TW, Hui E. Tranexamic acid: An important adjuvant in the treatment of melasma. J Cosmet Dermatol 2013;12:57-66.
[Google Scholar]
|
| 69. |
Cho HH, Choi M, Cho S, Lee JH. Role of oral tranexamic acid in melasma patients treated with IPL and low fluence QS nd: YAG laser. J Dermatolog Treat 2013;24:292-6.
[Google Scholar]
|
| 70. |
Wu S, Shi H, Wu H, Yan S, Guo J, Sun Y, et al. Treatment of melasma with oral administration of tranexamic acid. Aesthetic Plast Surg 2012;36:964-70.
[Google Scholar]
|
| 71. |
Lee HC, Thng TG, Goh CL. Oral tranexamic acid (TA) in the treatment of melasma: A retrospective analysis. J Am Acad Dermatol 2016;75:385-92.
[Google Scholar]
|
| 72. |
Padhi T, Pradhan S. Oral tranexamic acid with fluocinolone-based triple combination cream versus fluocinolone-based triple combination cream alone in melasma: An open labeled randomized comparative trial. Indian J Dermatol 2015;60:520.
[Google Scholar]
|
| 73. |
Hernández-Barrera R, Torres-Alvarez B, Castanedo-Cazares JP, Oros-Ovalle C, Moncada B. Solar elastosis and presence of mast cells as key features in the pathogenesis of melasma. Clin Exp Dermatol 2008;33:305-8.
[Google Scholar]
|
| 74. |
Iddamalgoda A, Le QT, Ito K, Tanaka K, Kojima H, Kido H, et al. Mast cell tryptase and photoaging: Possible involvement in the degradation of extra cellular matrix and basement membrane proteins. Arch Dermatol Res 2008;300 Suppl 1:S69-76.
[Google Scholar]
|
| 75. |
Grimbaldeston MA, Simpson A, Finlay-Jones JJ, Hart PH. The effect of ultraviolet radiation exposure on the prevalence of mast cells in human skin. Br J Dermatol 2003;148:300-6.
[Google Scholar]
|
| 76. |
Gonzalez S, Moran M, Kochevar IE. Chronic photodamage in skin of mast cell-deficient mice. Photochem Photobiol 1999;70:248-53.
[Google Scholar]
|
| 77. |
Crivellato E, Nico B, Ribatti D. Mast cells and tumour angiogenesis: New insight from experimental carcinogenesis. Cancer Lett 2008;269:1-6.
[Google Scholar]
|
| 78. |
Marone G, Columbo M, de Paulis A, Cirillo R, Giugliano R, Condorelli M. Physiological concentrations of zinc inhibit the release of histamine from human basophils and lung mast cells. Agents Actions 1986;18:103-6.
[Google Scholar]
|
| 79. |
Gupta M, Mahajan VK, Mehta KS, Chauhan PS. Zinc therapy in dermatology: A review. Dermatol Res Pract 2014;2014:709152.
[Google Scholar]
|
| 80. |
Passeron T. Melasma pathogenesis and influencing factors – An overview of the latest research. J Eur Acad Dermatol Venereol 2013;27 Suppl 1:5-6.
[Google Scholar]
|
| 81. |
Lee AY. Recent progress in melasma pathogenesis. Pigment Cell Melanoma Res 2015;28:648-60.
[Google Scholar]
|
| 82. |
Lieberman R, Moy L. Estrogen receptor expression in melasma: Results from facial skin of affected patients. J Drugs Dermatol 2008;7:463-5.
[Google Scholar]
|
| 83. |
Jang YH, Lee JY, Kang HY, Lee ES, Kim YC. Oestrogen and progesterone receptor expression in melasma: An immunohistochemical analysis. J Eur Acad Dermatol Venereol 2010;24:1312-6.
[Google Scholar]
|
| 84. |
Jian D, Jiang D, Su J, Chen W, Hu X, Kuang Y, et al. Diethylstilbestrol enhances melanogenesis via cAMP-PKA-mediating up-regulation of tyrosinase and MITF in mouse B16 melanoma cells. Steroids 2011;76:1297-304.
[Google Scholar]
|
| 85. |
Kim NH, Cheong KA, Lee TR, Lee AY. PDZK1 upregulation in estrogen-related hyperpigmentation in melasma. J Invest Dermatol 2012;132:2622-31.
[Google Scholar]
|
| 86. |
Kippenberger S, Loitsch S, Solano F, Bernd A, Kaufmann R. Quantification of tyrosinase, TRP-1, and trp-2 transcripts in human melanocytes by reverse transcriptase-competitive multiplex PCR – Regulation by steroid hormones. J Invest Dermatol 1998;110:364-7.
[Google Scholar]
|
| 87. |
Cohen PR. Melasma treatment: A novel approach using a topical agent that contains an anti-estrogen and a vascular endothelial growth factor inhibitor. Med Hypotheses 2017;101:1-5.
[Google Scholar]
|
| 88. |
Adalatkhah H, Sadeghi-Bazargani H. The first clinical experience on efficacy of topical flutamide on melasma compared with topical hydroquinone: A randomized clinical trial. Drug Des Devel Ther 2015;9:4219-25.
[Google Scholar]
|
| 89. |
Jagetia GC, Aggarwal BB. “Spicing up” of the immune system by curcumin. J Clin Immunol 2007;27:19-35.
[Google Scholar]
|
| 90. |
Ammon HP, Wahl MA. Pharmacology of Curcuma longa. Planta Med 1991;57:1-7.
[Google Scholar]
|
| 91. |
Tilak JC, Banerjee M, Mohan H, Devasagayam TP. Antioxidant availability of turmeric in relation to its medicinal and culinary uses. Phytother Res 2004;18:798-804.
[Google Scholar]
|
| 92. |
Hwang BM, Noh EM, Kim JS, Kim JM, You YO, Hwang JK, et al. Curcumin inhibits UVB-induced matrix metalloproteinase-1/3 expression by suppressing the MAPK-p38/JNK pathways in human dermal fibroblasts. Exp Dermatol 2013;22:371-4.
[Google Scholar]
|
| 93. |
Heng MC. Curcumin targeted signaling pathways: Basis for anti-photoaging and anti-carcinogenic therapy. Int J Dermatol 2010;49:608-22.
[Google Scholar]
|
| 94. |
Woo SH, Cho JS, Lee BS, Kim EK. Decolorization of melanin by lignin peroxidase from Phanerochaete chrysosporium. Biotechnol Bioprocess Eng 2004;9:256-60.
[Google Scholar]
|
| 95. |
Ollikka P, Alhonmäki K, Leppänen VM, Glumoff T, Raijola T, Suominen I, et al. Decolorization of azo, triphenyl methane, heterocyclic, and polymeric dyes by lignin peroxidase isoenzymes from phanerochaete chrysosporium. Appl Environ Microbiol 1993;59:4010-6.
[Google Scholar]
|
| 96. |
Mauricio T, Karmon Y, Khaiat A. A randomized and placebo-controlled study to compare the skin-lightening efficacy and safety of lignin peroxidase cream vs. 2% hydroquinone cream. J Cosmet Dermatol 2011;10:253-9.
[Google Scholar]
|
| 97. |
Smyth SS, McEver RP, Weyrich AS, Morrell CN, Hoffman MR, Arepally GM, et al. Platelet functions beyond hemostasis. J Thromb Haemost 2009;7:1759-66.
[Google Scholar]
|
| 98. |
Kim DS, Park SH, Park KC. Transforming growth factor-beta1 decreases melanin synthesis via delayed extracellular signal-regulated kinase activation. Int J Biochem Cell Biol 2004;36:1482-91.
[Google Scholar]
|
| 99. |
Cayırlı M, Calışkan E, Açıkgöz G, Erbil AH, Ertürk G. Regression of melasma with platelet-rich plasma treatment. Ann Dermatol 2014;26:401-2.
[Google Scholar]
|
| 100. |
Lima Ede A. Microneedling in facial recalcitrant melasma: Report of a series of 22 cases. An Bras Dermatol 2015;90:919-21.
[Google Scholar]
|
| 101. |
Schalka S. New data on hyperpigmentation disorders. J Eur Acad Dermatol Venereol 2017;31 Suppl 5:18-21.
[Google Scholar]
|
| 102. |
Teo WL, Gan E, Jinghan A, Chuah SY, Alain K, Goh CLet al. Double blind placebo controlled trial to evaluate of the effectiveness of a dietary supplement rich in carotenoids as adjunct to topical lightening cream for the treatment of melasma: A pilot study. J Pigmentery Disord 2015;2:164.
[Google Scholar]
|
Fulltext Views
39,239
PDF downloads
11,332





