Translate this page into:
In vitro antimicrobial susceptibility of Mycoplasma hominis genital isolates
2 Department of Molecular and Cellular Biology and Pathology "Luigi Califano", Medicine School, University of Napoli, Napoli, Italy
Correspondence Address:
Salvatore Pignanelli
via Guelfa n. 30, Bologna
Italy
| How to cite this article: Pignanelli S, Pulcrano G, Schiavone P, Iula VD, Catania MR. In vitro antimicrobial susceptibility of Mycoplasma hominis genital isolates. Indian J Dermatol Venereol Leprol 2015;81:286-288 |
Sir,
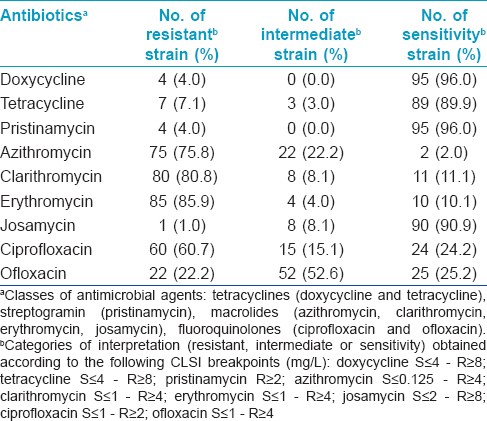
Mycoplasma hominis may be implicated in several diseases. [1] Data on the prevalence and the antimicrobial resistance of M. hominis from various countries are few and controversial. [1] We investigated the antimicrobial susceptibility of M. hominis from cervical and urethral swabs of outpatients in Northern and Southern Italy. A comparison of these data was done with similar studies worldwide, from 2011, to investigate the prevalence, therapeutic management and spread of antimicrobial resistance of this atypical pathogen. In two Italian hospitals of northern (Imola) and southern (Naples) Italy, a total of 2480 patients (1980 women and 500 men) aged 18-40, with cervicitis/urethritis, were examined from July 2009 to December 2013. For each patient, two swabs from either the uterine cervix or urethra were collected and processed. The detection and the antimicrobial susceptibility testing (AST) of M. hominis genital isolates were performed using the Mycoplasma IST2 kit (bioMerieux, Marcy-l′Etoile, France). The techniques used for inoculation, diagnostic criteria and statistical analysis have been described earlier. [1] M. hominis was detected in 99 (4%) biological samples (84/1980 in women and 15/500 in men); significant bacterial load was revealed in 82 (3.3%). Colonization and infection by M. hominis decreased with increasing age (P < 0.001). AST was performed against all bacterial isolates. Tetracyclines exhibited a sensitivity percentage of 92.9%, streptogramins 96.0%, macrolides 28.5%, and fluoroquinolones 24.7% [Table - 1].
Articles on the prevalence and antimicrobial susceptibility of M. hominis from sexually active population published from 2011 were accessed on PubMed, from which we selected 17 articles from South America, Africa, Europe, and Far East. [2],[3],[4],[5],[6],[7],[8],[9],[10],[11],[12],[13],[14],[15],[16],[17],[18] Only 10 (58.8%) of these focused their attention on antimicrobial susceptibility. [3],[4],[5],[6],[7],[8],[9],[10],[13],[15] Moreover, 9 (52.9%) articles studied female patients, [2],[3],[4],[5],[6],[9],[11],[15],[18] 4 (23.5%) male, [7],[14],[16],[17] and 4 (23.5%) both; [8],[10],[12],[13] the populations showed genital disorder in 15 (88.2%) cases. [2],[4],[5],[6],[7],[8],[9],[10],[11],[12],[13],[14],[15],[16],[18] In these works, the prevalence of M. hominis ranged from 0.5% to 39.7% (male 0.5-28.3% and female 0.9-39.7%). [2],[3],[4],[5],[6],[7],[8],[9],[10],[11],[12],[13],[14],[15],[16],[17] Studies from South Africa showed high prevalence (39.7%), [5] followed by Paraguay (30.9%), [2] Cuba (31%), [4] Russia (28.3%), [14] Romania (21.9%), [18] China (15.6%), [15] Japan (6.0%), [16] Italy (1.1-6.9%), [7],[8],[9],[10] Poland (4.0%), [11] Burkina Faso (2.7%), [6] and Hungary (0.9-1.2%). [12],[13] In addition, two studies on asymptomatic patients displayed a prevalence of 7.5% in Romania and 6.3% in Japan. [3],[17] The antimicrobial resistances of M. hominis genital isolates from different countries, briefly described in [Table - 2], was as follows: high resistance to macrolides (98% South Africa, 88% Cuba, 72.4% Italy, 71.5% Paraguay, 68% Burkina Faso, 63.4% China, 54.8% Hungary, and 34.7% Romania), [3],[4],[5],[6],[7],[8],[9],[10],[12],[13],[15] moderate to fluoroquinolones (67.6% China, 50% Romania, 50% Cuba, 43% Paraguay, 26% Burkina Faso, 25% Italy, 25% South Africa, and 8% Hungary), [3],[4],[5],[6],[7],[8],[9],[10],[12],[13],[15] low to streptogramin (10% Romania and 0% Italy). [3],[4],[5],[6],[7],[8],[9],[10] Resistance to tetracyclines varied significantly (95% South Africa, 49% Cuba, 33.5% Burkina Faso, 28.7% Paraguay, 12.5% Romania, 8% Hungary, 4.2% Italy, and 1.8% China). [3],[4],[5],[6],[7],[8],[9],[10],[12],[13],[15]
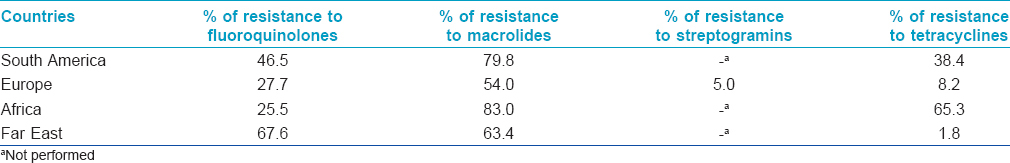
In the present study, M. hominis emerged as a clinically significant pathogen in 82 (3.3%) sexually active patients. The prevalence varies in various countries, although epidemiological similarities are found in adjacent geographic areas (P < 0.001). In symptomatic populations, almost homogeneous data of prevalence were found in South America and Europe [2],[4],[7],[8],[9],[10],[11],[12],[13],[18] , which could be explained by similar social and cultural characteristics, which could also explain significant differences in the prevalence of M. hominis detected in Africa. [5],[6] Data from the Far East were not comparable due to differences in patient selection.
In our study, streptogramins showed the best activity against M. hominis, followed by tetracyclines, josamycin, fluoroquinolones, and finally by other macrolides [Table - 1]. Our results were quite similar to European results and revealed that, except macrolides, antimicrobial agents within the same class exhibited similar antimicrobial activity against M. hominis. [Table - 1]. Resistance to macrolides among M. hominis is an ongoing phenomenon with significant implications in clinical practice, as described for other genital mycoplasmas. [1] The exception to this is josamycin, which shows good activity. Our data showed that M. hominis infections in non-pregnant women could be treated with tetracyclines, streptogramins or josamycin during pregnancy and in newborns, our results suggest the use of streptogramins (pristinamycin) or josamycin, as tetracyclines are contraindicated. Pristinamycin and josamycin are structurally different but functionally similar and are grouped in the macrolide-lincosamide-streptogramin B class of antibiotics which all bind to the 50S ribosomal subunit. The most interesting data are related to good activity of streptogramins, even though confined to European studies. [3],[4],[5],[6],[7],[8],[9],[10] This finding is very important because, if confirmed, it would add a safe and effective class of antimicrobial agents in pregnant women and neonates. Further studies on the worldwide prevalence and antimicrobial resistance of M. hominis are important to refine therapeutic strategies against this atypical pathogen.
| 1. |
Pignanelli S, Pulcrano G, Iula VD, Zaccherini P, Testa A, Catania MR. In vitro antimicrobial profile of Ureaplasma urealyticum from genital tract of childbearing-aged women in Northern and Southern Italy. APMIS 2014;122:552-5.
[Google Scholar]
|
| 2. |
Mendoza L, Mongelos P, Paez M, Castro A, Rodriguez-Riveros I, Gimenez G, et al. Human papillomavirus and other genital infections in indigenous women from Paraguay: A cross-sectional analytical study. BMC Infect Dis 2013;13:531.
[Google Scholar]
|
| 3. |
Mihai M, Valentin N, Bogdan D, Carmen CM, Coralia B, Demetra S. Antibiotic susceptibility profiles of Mycoplasma hominis and ureaplasma urealyticum isolated during a population-based study concerning women infertility in northeast romania. Braz J Microbiol 2011;42:256-60.
[Google Scholar]
|
| 4. |
Díaz L, Cabrera LE, Fernández T, Ibáñez I, Torres Y, Obregón Y, et al. Frequency and antimicrobial sensitivity of Ureaplasma urealyticum and Mycoplasma hominis in patients with vaginal discharge. MEDICC Rev 2013;15:45-7.
[Google Scholar]
|
| 5. |
Redelinghuys MJ, Ehlers MM, Dreyer AW, Lombaard HA, Kock MM. Antimicrobial susceptibility patterns of Ureaplasma species and Mycoplasma hominis in pregnant women. BMC Infect Dis 2014;14:171.
[Google Scholar]
|
| 6. |
Karou SD, Djigma F, Sagna T, Nadembega C, Zeba M, Kabre A, et al. Antimicrobial resistance of abnormal vaginal discharges microorganisms in Ouagadougou, Burkina Faso. Asian Pac J Trop Biomed 2012;2:294-7.
[Google Scholar]
|
| 7. |
Messano GA, Petti S. Antibiotic resistance as a public health problem: The case of genital mycoplasmoses. Ig Sanita Pubbl 2011;67:697-706.
[Google Scholar]
|
| 8. |
De Francesco MA, Caracciolo S, Bonfanti C, Manca N. Incidence and antibiotic susceptibility of Mycoplasma hominis and Ureaplasma urealyticum isolated in Brescia, Italy, over 7 years. J Infect Chemother 2013;19:621-7.
[Google Scholar]
|
| 9. |
Leli C, Meucci M, Vento S, D'Alò F, Farinelli S, Perito S, et al. Microbial and vaginal determinants influencing Mycoplasma hominis and Ureaplasma urealyticum genital colonization in a population of female patients. Infez Med 2013;21:201-6.
et al. Microbial and vaginal determinants influencing Mycoplasma hominis and Ureaplasma urealyticum genital colonization in a population of female patients. Infez Med 2013;21:201-6.'>[Google Scholar]
|
| 10. |
Leli C, Mencacci A, Bombaci JC, D'Alò F, Farinelli S, Vitali M, et al. Prevalence and antimicrobial susceptibility of Ureaplasma urealyticum and Mycoplasma hominis in a population of Italian and immigrant outpatients. Infez Med 2012;20:82-7.
et al. Prevalence and antimicrobial susceptibility of Ureaplasma urealyticum and Mycoplasma hominis in a population of Italian and immigrant outpatients. Infez Med 2012;20:82-7.'>[Google Scholar]
|
| 11. |
Tomusiak A, Heczko PB, Janeczko J, Adamski P, Pilarczyk-Zurek M, Strus M. Bacterial infections of the lower genital tract in fertile and infertile women from the southeastern Poland. Ginekol Pol 2013;84:352-8.
[Google Scholar]
|
| 12. |
Pónyai K, Mihalik N, Ostorházi E, Farkas B, Párducz L, Marschalkó M, et al. Incidence and antibiotic susceptibility of genital mycoplasmas in sexually active individuals in Hungary. Eur J Clin Microbiol Infect Dis 2013;32:1423-6.
[Google Scholar]
|
| 13. |
Farkas B, Ostorházi E, Pónyai K, Tóth B, Adlan E, Párducz L, et al. Frequency and antibiotic resistance of Ureaplasma urealyticum and Mycoplasma hominis in genital samples of sexually active individuals. Orv Hetil 2011;152:1698-702.
[Google Scholar]
|
| 14. |
Andreeva IV, Kozlov SN, Korolev SV, Belikov AN, Grinev AV, Evstaf'ev VV, et al. Diagnostic and treatment patterns in management of male patients with nongonococcal urethritis: Results of Russian multicentral cross-sectional study. Antibiot Khimioter 2012;57:32-40.
et al. Diagnostic and treatment patterns in management of male patients with nongonococcal urethritis: Results of Russian multicentral cross-sectional study. Antibiot Khimioter 2012;57:32-40.'>[Google Scholar]
|
| 15. |
Zhu C, Liu J, Ling Y, Dong C, Wu T, Yu X, et al. Prevalence and antimicrobial susceptibility of Ureapl-asma urealyticum and Mycoplasma hominis in Chinese women with genital infectious diseases. Indian J Dermatol Venereol Leprol 2012;78:406-7.
[Google Scholar]
|
| 16. |
Ito S, Tsuchiya T, Yasuda M, Yokoi S, Nakano M, Deguchi T. Prevalence of genital mycoplasmas and ureaplasmas in men younger than 40 years-of-age with acute epididymitis. Int J Urol 2012;19:234-8.
[Google Scholar]
|
| 17. |
Nakashima K, Shigehara K, Kawaguchi S, Wakatsuki A, Kobori Y, Nakashima K, et al. Prevalence of human papillomavirus infection in the oropharynx and urine among sexually active men: A comparative study of infection by papillomavirus and other organisms, including Neisseria gonorrhoeae, Chlamydia trachomatis, Mycoplasma spp., and Ureaplasma spp. BMC Infect Dis 2014;14:43.
[Google Scholar]
|
| 18. |
Rodrigues MM, Fernandes PÁ, Haddad JP, Paiva MC, Souza Mdo C, Andrade TC, et al. Frequency of Chlamydia trachomatis, Neisseria gonorrhoeae, Mycoplasma genitalium, Mycoplasma hominis and Ureaplasma species in cervical samples. J Obstet Gynaecol 2011;31:237-41.
[Google Scholar]
|
Fulltext Views
3,084
PDF downloads
1,678





