Translate this page into:
Lack of efficacy of liposomal glucantime in the treatment of cutaneous leishmaniasis
2 Biotechnology Research Center, Nanotechnology Research Center, School of Pharmacy, Mashhad University of Medical Sciences, Mashhad, Iran
3 Health Sciences Research Center, Department of Biostatistics and Epidemiology, School of Health, Mashhad University of Medical Sciences, Mashhad, Iran
Correspondence Address:
Bita Kiafar
Cutaneous Leishmaniasis Research Center, Department of Dermatology, School of Medicine, Imam Reza Hospital, Mashhad University of Medical Sciences, Mashhad
Iran
| How to cite this article: Ghoyonlo VM, Jafari MR, Yazdanpanah MJ, Esmaili H, Noori S, Kiafar B. Lack of efficacy of liposomal glucantime in the treatment of cutaneous leishmaniasis. Indian J Dermatol Venereol Leprol 2016;82:347-349 |
Sir,
Pentavalent antimony compounds are known to be the main anti-leishmania modalities prescribed either intralesionally or systemically, although pain due to injection and systemic toxicities pose major limitations to their use. Liposomal formulations are among the new methods used to enhance transcutaneous absorption, increase penetration into the macrophages and delay clearance from the site of action.[1],[2],[3] In this pilot study, we sought to evaluate the efficacy and safety of a new topical formulation of liposomal meglumine antimoniate for the treatment of old world cutaneous leishmaniasis.
The study was approved by the ethical committee of the Mashhad University of Medical Science. Twenty patients with cutaneous leishmaniasis attending the leishmaniasis clinic of Imam Reza Hospital were enrolled in this study based on the following inclusion criteria: (1) parasitological confirmation of the disease (2) no previous history of treatment (3) duration of disease <6 months (4) largest diameter of the lesion <80 mm (5) refusal to receive conventional intralesional injection of glucantime and (6) informed consent. Exclusion criteria were: (1) antimoniate sensitivity (2) irregular attendance (3) secondary infection of the lesion (4) concomitant use of other treatments.
Liposomal meglumine antimoniate was prepared locally using small unilamellar liposomes with an approximate diameter of 110 nanometers by fusion method. Shape and sizes of the nanoliposomes were checked by particle size analyzer. Furthermore, the particles were checked for stability, zeta potential and meglumine antimonite content. Patients were asked to apply the formulation 3 times a day for eight weeks. Follow-up visits were scheduled weekly and two largest perpendicular diameters of the lesion in millimeters were recorded. Potential local adverse effects of the treatment were also recorded.
After 8 weeks of the treatment, decrease in induration was recorded and clinical efficacy of the formulation was defined as: (1) significant response (>75% decrement) (2) moderate response (50–75% decrement) (3) mild response (25–50% decrement)(4) slight response (<25% decrement) (5) no response (no change or increase of indurated area). Patients were followed up at 1.5, 3 and 6 months after the end of the treatment to evaluate disease progression and suspected adverse effects. All the data was analyzed with SPSS version 11.5 (IBM Corp., Armonk, NY) for Windows, New York, USA. Eight men and 12 women were enrolled and a total of 55 lesions were studied. The mean age (= SD) of patients enrolled was: 23.2 ± 18.48 years. (range 2-52 years). Initial induration was calculated as the product of two perpendicular diagonals of the lesion. Accordingly, the largest lesion was 4118 mm 2 and the smallest was 12 mm 2 (mean 349.85 mm 2). In four patients, allergic contact dermatitis to liposomal glucantime occurred at the treatment site. Among them, two patients showed adverse reactions at week 6 of treatment resulting in treatment withdrawal. and two other patients at weeks and at week 3 respectively. In the last patient the dermatitis resolved with continued treatment. Three patients were excluded from the study, two patients with a total of 19 lesions because of local adverse effects and one patient with a single lesion because of irregular attendance. Finally, 17 patients with a total lesion count of 35 were statically analyzed. Since the improvements calculated as mentioned above did not have a normal distribution, non- parametric tests such as Kruskal–Wallis and Spearman's test were used to analyze the data.
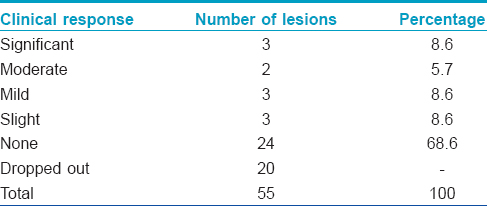
Clinical response rates of the lesions at 8 weeks are summarized in [Table - 1]. The most common outcome was no response which was noted in 24 (68.6%) lesions. Significant response was noted in only 3 (8.6%) lesions.
Spearman's test showed no statistically significant correlation between the duration of the lesion and response rate and between the initial size of the lesion and improvement rate (r = 0.117, P = 0.505). Contrary to our expectation, in this pilot study liposomal formulation of glucantime was not effective in the treatment of cutaneous leishmaniasis. The lack of efficacy could be due to the low penetration of the formulation through skin considering that most treated lesions were not ulcerated. This was unexpected since penetration of the formulation and its efficacy in the treatment of cutaneous leishmaniasis in BALB/c mice has been previously studied.[4] However, it failed to be clinically effective in humans in this pilot study, in contrast to liposomal amphotericin which was effective in another clinical trial reported from our country.[5] Instability of the drug in these formulation is also another concern. The main limitations of this study are the small sample size and lack of a control group.
Acknowledgment
Special thanks to Miss. Akram Momenzadeh for her assistance in preparing and submitting this manuscript.
Financial support and sponsorship
This article was extracted from the thesis prepared by Dr. Soleiman Noori. The research council of the Mashhad University of Medical Sciences, Mashhad, Iran is appreciated for financially supporting this study, thesis number T-2890.
Conflicts of interest
There are no conflicts of interest.
| 1. |
Carneiro G, Aguiar MG, Fernandes AP, Ferreira LA. Drug delivery systems for the topical treatment of cutaneous leishmaniasis. Expert Opin Drug Deliv 2012;9:1083-97.
[Google Scholar]
|
| 2. |
Momeni A, Rasoolian M, Momeni A, Navaei A, Emami S, Shaker Z, et al. Development of liposomes loaded with anti-leishmanial drugs for the treatment of cutaneous leishmaniasis. J Liposome Res 2013;23:134-44.
[Google Scholar]
|
| 3. |
Barros NB, Migliaccio V, Facundo VA, Ciancaglini P, Stábeli RG, Nicolete R, et al. Liposomal-lupane system as alternative chemotherapy against cutaneous leishmaniasis: Macrophage as target cell. Exp Parasitol 2013;135:337-43.
[Google Scholar]
|
| 4. |
Kalat SA, Khamesipour A, Bavarsad N, Fallah M, Khashayarmanesh Z, Feizi E, et al. Use of topical liposomes containing meglumine antimoniate (Glucantime) for the treatment of L. major lesion in BALB/c mice. Exp Parasitol 2014;143:5-10.
[Google Scholar]
|
| 5. |
Layegh P, Rajabi O, Jafari MR, Emamgholi Tabar Malekshah P, Moghiman T, Ashraf H, et al. Efficacy of topical liposomal amphotericin B versus intralesional meglumine antimoniate (Glucantime) in the treatment of cutaneous leishmaniasis. J Parasitol Res 2011;2011:656523.
[Google Scholar]
|
Fulltext Views
2,673
PDF downloads
2,599





