Translate this page into:
Local antimicrobial, protease and cytokine defense systems in psoriatic skin
2 Department of Infectology and Dermatology, Riga Stradins University, Riga, Latvia
Correspondence Address:
Elga Sidhom
Kronvalda Bulvaris 9, Riga, LV - 1010
Latvia
| How to cite this article: Sidhom E, Pilmane M, Kisis J. Local antimicrobial, protease and cytokine defense systems in psoriatic skin. Indian J Dermatol Venereol Leprol 2016;82:284-291 |
Abstract
Background: Psoriasis vulgaris is an inflammatory skin condition characterized by dramatic biochemical and immunological changes. Aims: The aim of the study was to evaluate antimicrobial response, tissue degeneration reactions and distribution of inflammatory cytokines in untreated psoriatic skin as well as the correlations between these factors and influence on the course of the disease. Methods: We evaluated skin samples obtained from routine punch biopsies in 40 patients with psoriasis vulgaris. All tissue specimens were examined by hematoxylin and eosin staining and immunohistochemistry for human beta defensin 2 (HBD-2), matrix metalloproteinase 2 (MMP-2), tumor necrosis factor-alpha (TNF-alpha), interleukin 6 (IL-6) and IL-8. The staining intensity was semi-quantitatively graded. Results: Numerous keratinocytes, fibroblasts and macrophages expressed HBD-2 while the number of MMP-2-positive macrophages, fibroblasts and epitheliocytes varied. TNF-alpha-positive cells varied from a few to numerous in each microscopic field. IL-6-positive cells varied from a few to abundant and IL-8-positive cells from numerous to abundant in each field. Limitations: This study had a rather small patient number. Conclusions: Psoriatic skin shows a strong correlative increase in skin antimicrobial proteins and enzymes mediating tissue degeneration suggesting that the skin maintains compensatory mechanisms during persistent remodeling. While individual notable decrease in antimicrobial proteins was observed in some tissue samples, generally the increased human beta defensin associated with psoriasis is likely to be due to an altered immune status. TNF-alpha, IL-6 and IL-8 are common cytokines expressed in psoriatic skin plaques to maintain the inflammatory cycle. HBD-2, MMP-2 and TNF-alpha positively correlate with the severity of psoriasis. Meanwhile, the expression of IL-8 significantly decreases with clinically more severe psoriasis, perhaps making these factors candidate prognostic factors for psoriatic inflammation.Introduction
Psoriasis vulgaris is an inflammatory condition of the skin with possible nail and joint involvement associated with immense biochemical and immunological changes. The pathogenesis of psoriasis is still unclear but T cells are thought to play a primary role. In addition, psoriatic skin classically presents with abnormal epidermal changes and modified keratinocytes.[1] These abnormal keratinocytes produce excess amounts of antimicrobial peptides and proteins and induce immune cells to produce inflammatory cytokines. One of the antimicrobial peptides that has been isolated from psoriatic scales is human beta defensin 2 (HBD-2), a cationic microbial peptide expressed only by induction with pro-inflammatory cytokines such as tumor necrosis factor-alpha (TNF-alpha) or interleukin 8 (IL-8). TNF-alpha is highly expressed in psoriatic lesions and induces increased HBD-2 expression in keratinocytes. HBD-2 is chemoattractive to macrophages and interacts with other inflammatory cells.[2],[3] Antimicrobial peptides play an essential role in permeability barrier homeostasis highlighting their function as a chemical shield of the skin and a physical barrier. In addition to keratinocytes, the natural microflora of the skin participate in barrier defense by expressing antimicrobial molecules. Upregulation of HBD-2 in psoriatic skin is thought to contribute to pathogenesis and the resistance to secondary infections caused by Gram-negative bacteria or candida. Thus, HBD-2 is a dynamic component of the local epidermal defense system.[4],[5]
Matrix metalloproteinase 2 (MMP-2) is a gelatinase that is a zinc-dependent endopeptidase involved in the remodeling of extracellular matrix. Gelatinases have been found to be important in wound healing, angiogenesis, morphogenesis, metastasis and tumor invasion. MMP-2 has a unique structure and in its active form, can bind to gelatin. MMP-2 and its tissue inhibitor are overexpressed in the suprabasal layers of psoriatic epidermis.[6]
Interleukin-6 is a multifunctional cytokine produced by various cells (fibroblasts, macrophages, monocytes, endothelial cells) and participates in host defense, immune responses, hematopoiesis and acute and chronic inflammatory reactions. It can promote the activation of lymphocytes, myeloid cells and epidermal keratinocytes resulting in increased inflammation.
Interleukin-8 is secreted by epithelial, endothelial, mesothelial and tumor cells, fibroblasts, monocytes and neutrophils as an inflammatory response. IL-8 plays a role in inflammation and wound healing; it promotes chemotaxis of all migratory immune cells, recruits T cells to the site of inflammation via neutrophil activation and has potent angiogenic, pro-inflammatory, growth-promoting and matrix degradative properties. TNF-alpha strongly induces IL-8 production that, with Type I interferons and interferon-gamma, is one of the key cytokines in psoriatic inflammation.
Tumor necrosis factor-alpha is a pro-inflammatory cytokine produced by T cells induced by myeloid dermal dendritic cells in psoriatic lesions and reciprocally TNF-alpha activates dendritic cells. TNF-alpha also has its own anti-inflammatory properties; in some patients, anti-TNF therapy can induce new onset of psoriasis. Local and systemic overexpression of pro-inflammatory cytokines IL-6, IL-8 and TNF-alpha are involved in the initiation, maintenance, course, severity and recurrence of psoriasis.[1],[7],[8] Therefore, the role of cytokines in the pathogenesis of psoriasis has resulted in their frequent use in new therapeutic approaches that directly target inflammatory events but finding an effective treatment is an ongoing challenge.
The aim of the study was to evaluate antimicrobial response, tissue degeneration reaction and distribution of inflammatory cytokines in untreated psoriatic skin as well as the correlations between these factors and influence on the course of the disease.
Methods
Patients
We selected 40 patients with psoriasis between the ages of 18 and 70 who had the disease for at least 6 weeks and presented with characteristic eruptions at typical sites. All patients had a clinically and histologically confirmed diagnosis of psoriasis vulgaris. Patients who had a severe tan, had previously received local and systemic medication or had concomitant diseases were excluded. Skin biopsies of new untreated psoriatic lesions were obtained using a routine 3 mm punch following local anesthesia with lidocaine. Ten clinically healthy skin samples obtained during nevus excision procedure were used as control material.
The study was approved by the ethical committee at Riga Stradins University, permit issued on September 10, 2009.
Methods
Skin biopsy tissue was fixed in Stefanini's solution, dehydrated and embedded in paraffin.[9] Four micrometer thick sections were prepared and stained with hematoxylin and eosin.[10]
Immunohistochemistry (IHC)
Human beta defensin 2 (Cat No AF2758, Lot No VJU01, obtained from goat, 1:100 dilution, R&D Systems, Germany), MMP-2 (Cat No AF902, Lot No DUB03, obtained from goat, 1:100 dilution, R&D Systems, Germany), TNF-alpha (code ab 6671, obtained from rabbit, 1:100 dilution, Abcam, Cambridge, UK), IL-6 (NYRhIL6: Sc-73319, obtained from mouse, 1:50 dilution, Santa Cruz Biotechnology, Inc., USA) and IL-8 (C-19: Sc-1269, obtained from goat, 1:50 dilution, Santa Cruz Biotechnology, Inc., USA) primary antibodies were used in a standard biotin–streptavidin immunohistochemistry process.[11] Skin biopsy tissues were deparaffinized, washed in alcohol and water, washed for 10 min in wash buffer (tris-buffered saline) and microwaved in boiling EDTA buffer for 5 min. The samples were allowed to cool and washed twice in wash buffer for 5 min. Samples were blocked with normal blocking sera for 20 min to decrease background staining. All samples were stained with primary antibodies for 1 h, washed for 10 min in wash buffer and then stained for 30 min with Dako REAL ™ Detection System (LSAB ™+) with biotin-related secondary antibodies (code K1015, DakoCytomation, Denmark) and washed again for 5 min in wash buffer. We further stained our samples for 25 min with LSAB ™ + with peroxidase-labeled streptavidin (code K0690, DakoCytomation, Denmark), washed for 5 min in wash buffer and processed for 10 min with DAB substrate-chromogen system (code K3468, DakoCytomation, Denmark) to stain positive structures brown. Samples were then rinsed in running water and counterstained with hematoxylin.
Samples were examined on a Leica DC 300F camera and image processing and analysis was performed on Image Pro Plus 6.0 software (Media Cybernetics, Silver Spring, Maryland, USA).
The intensity of immunostaining was semiquantitatively graded, as follows:[12]
- A few positive structures in the visual field at magnification of ×400 were labeled +
- A moderate number of positive structures in the visual field at magnification of ×400 was labeled ++
- Numerous positive structures in the visual field at magnification of ×400 were labeled +++
- An abundance of positive structures in the visual field at magnification of ×400 was marked ++++.
For statistical analyses, we used non-parametric statistics and Spearman's rank correlation coefficient.[13]
Results
The psoriatic epidermis of our tissue samples was characterized by loss of the granular cell layer, parakeratosis, orthokeratosis and acanthosis as well as the formation of the characteristic Munro microabscesses. We observed increased mitotic activity in basal cells. Polymorphonuclear leukocytes and lymphocytes infiltrated dermis and epidermis. The arterioles within the papillary dermis were slightly dilated and showed sclerotic changes.
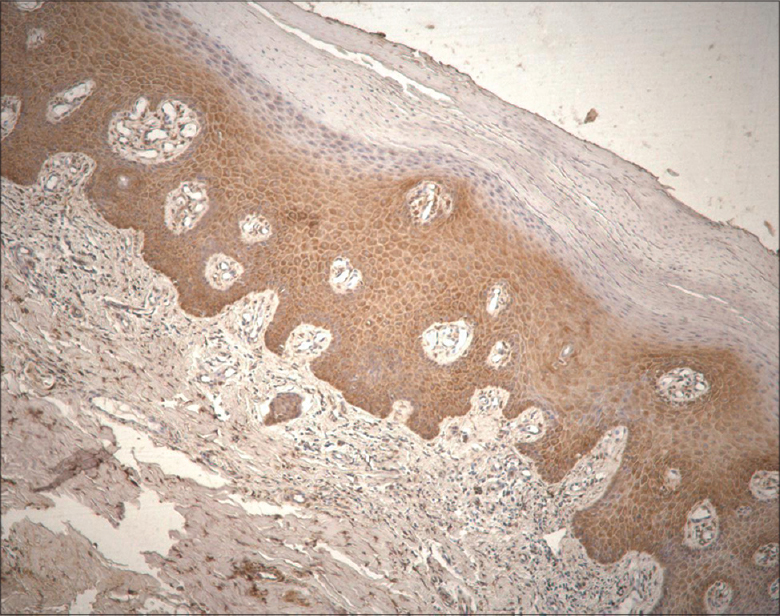
Numerous (+++) to abundant (++++) keratinocytes, fibroblasts and macrophages expressed HBD-2 with more pronounced expression in sites of well-defined inflammation [Figure - 1] and [Table - 1].
 |
| Figure 1: Abundance of human beta defensin 2-containing keratinocytes and connective tissue cells in psoriatic skin lesion (HBD-2 IHC, ×200) |
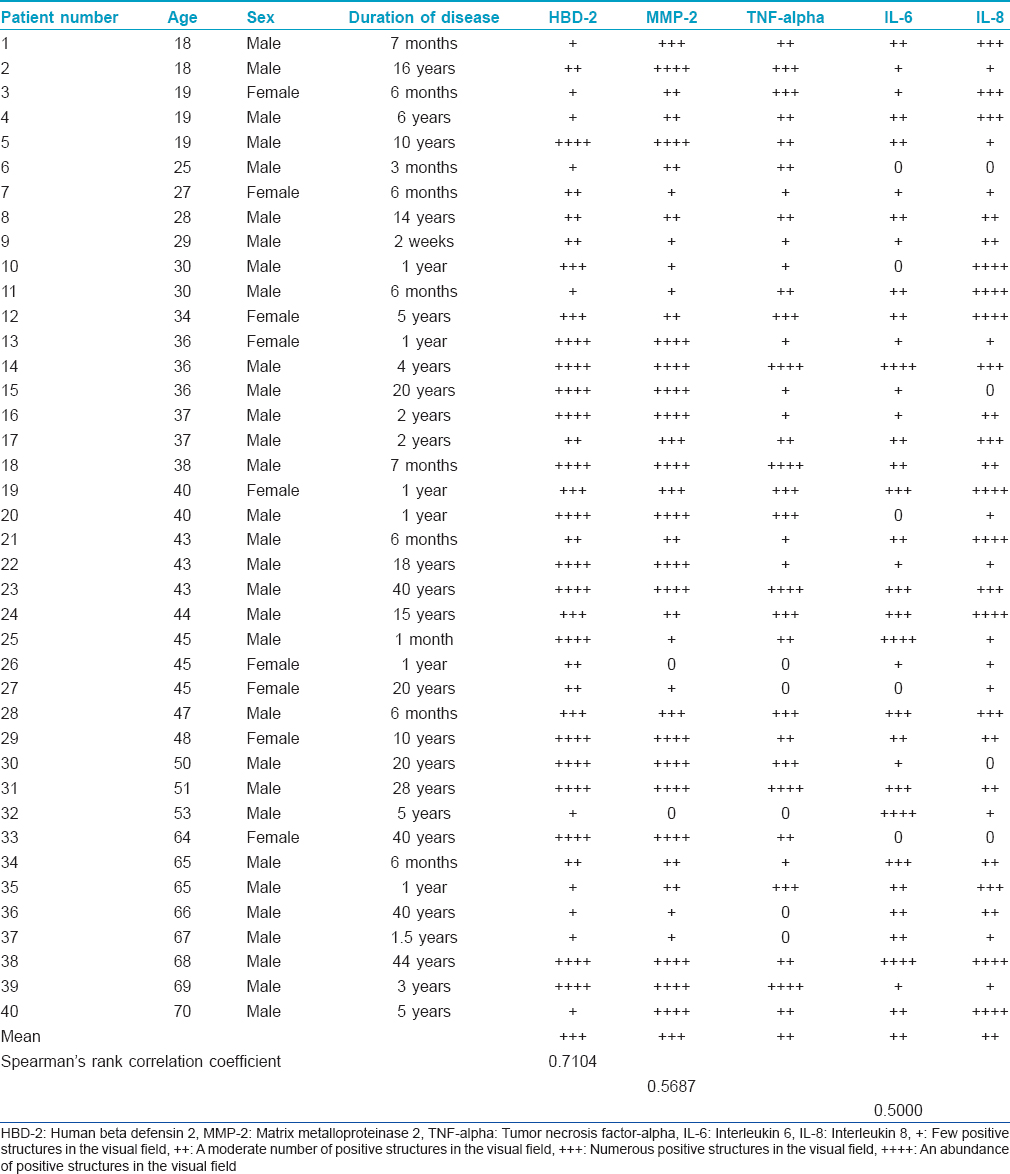
The number of MMP-2 positive macrophages, fibroblasts and keratinocytes varied from a few (+) to abundant (++++), mainly in the dermis of psoriatic skin [Figure - 2].
 |
| Figure 2: Numerous MMP-2-expressing cells in the epidermis and papillary dermis of psoriatic patient skin (MMP-2 IHC, ×200) |
We found TNF-alpha-positive lymphocytes and macrophages in almost all skin samples, particularly in the papillary dermis, walls of blood vessels and eccrine sweat glands. The number of TNF-alpha-positive cells varied from a few (+) to numerous (+++) in each visual field [Figure - 3].
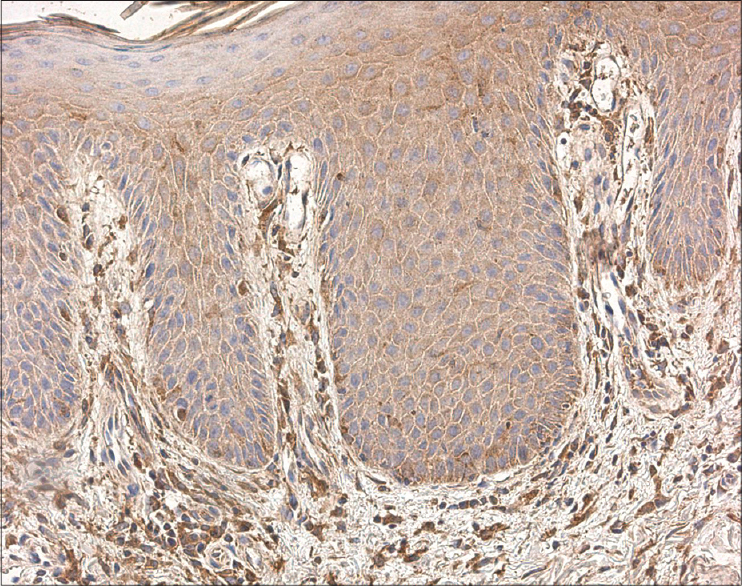 |
| Figure 3: Numerous TNF-alpha positive inflammatory cells, particularly in psoriasis, populated the papillary dermis (TNF-alpha IHC, ×250) |
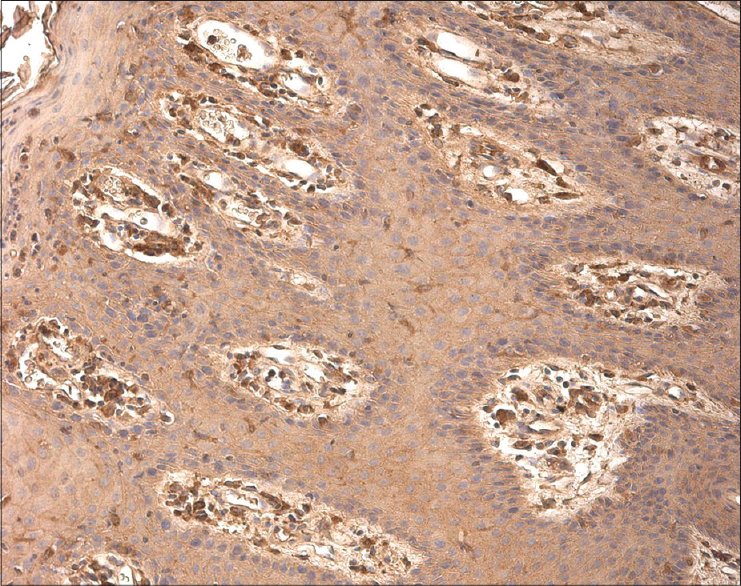
We detected IL-6-positive cells ranging from a few (+) to abundant (++++) in each visual field [Figure - 4], while IL-8-positive cells were numerous (+++) to abundant (++++) in each visual field. We detected IL-8 in the epidermis and dermis, inflammatory infiltrates, hair follicles and surrounding blood vessels [Figure - 5].
 |
| Figure 4: Numerous IL-6 positive cells throughout the epidermal and dermal layers of psoriatic skin (IL-6 IHC, ×100) |
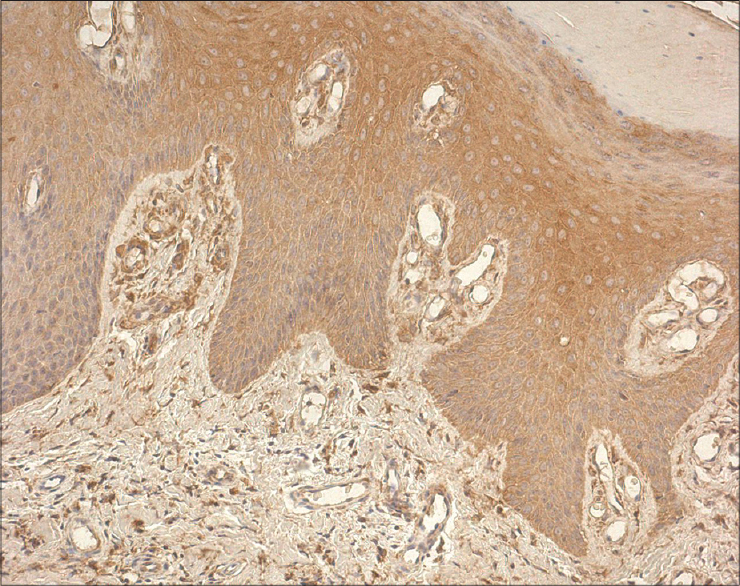 |
| Figure 5: Marked expression of IL-8 in the epidermis and dermal inflammatory cells (IL-8 IHC, ×200) |
Healthy skin samples obtained during nevus excision procedure as negative controls are shown in [Figure - 6], [Figure - 7], [Figure - 8], [Figure - 9], [Figure - 10].
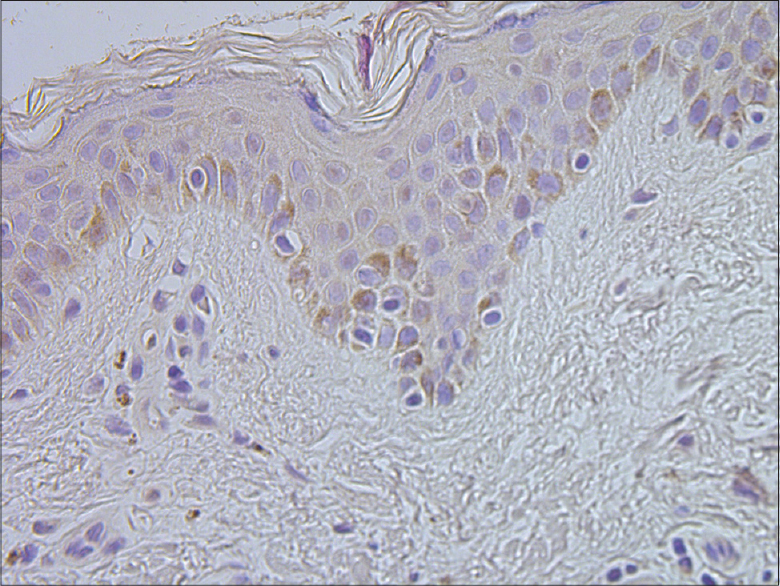 |
| Figure 6: Basal keratinocytes and sparse connective tissue cells containing human beta defensin 2 in healthy skin (HBD-2 IHC, ×400) |
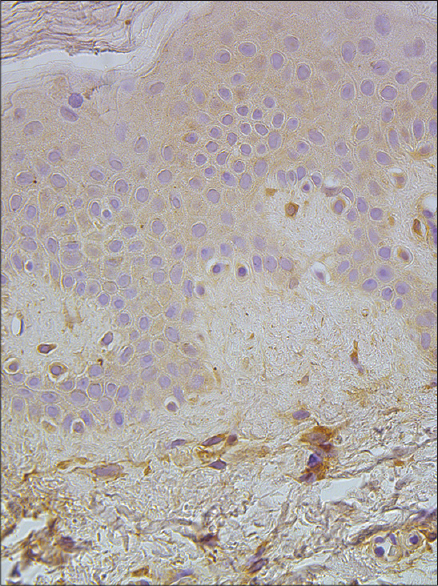 |
| Figure 7: A few to a moderate number of MMP-2-expressing cells in the epidermis and papillary dermis of healthy skin (MMP-2 IHC, ×400) |
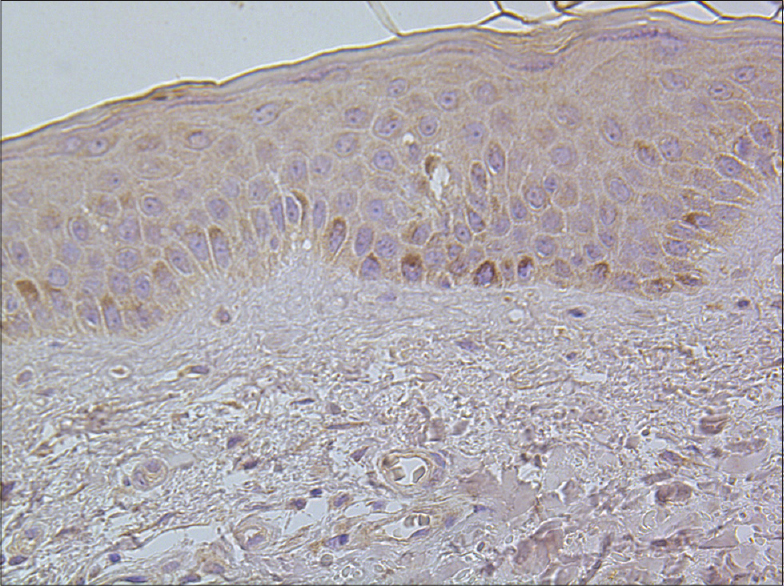 |
| Figure 8: Separate TNF-alpha positive basal keratinocytes in healthy skin tissue (TNF-alpha IHC, ×400) |
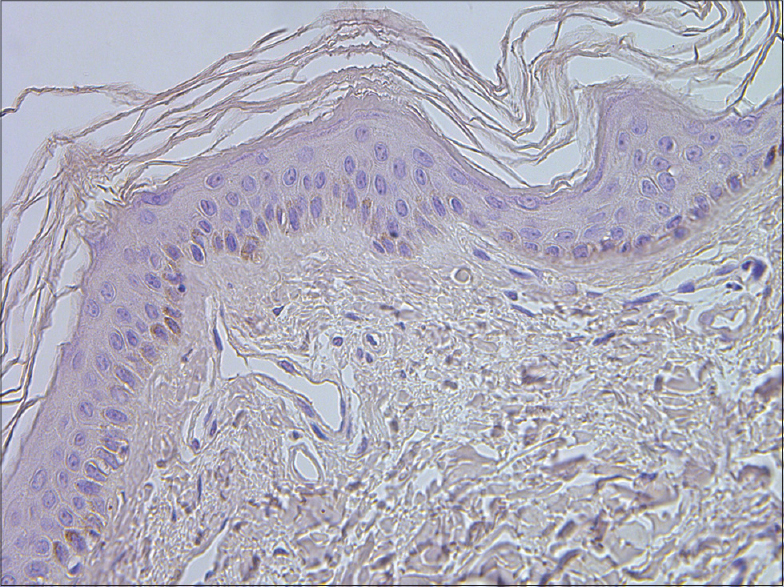 |
| Figure 9: A few IL-6 positive cells in epidermis of healthy skin (IL-6 IHC, ×400) |
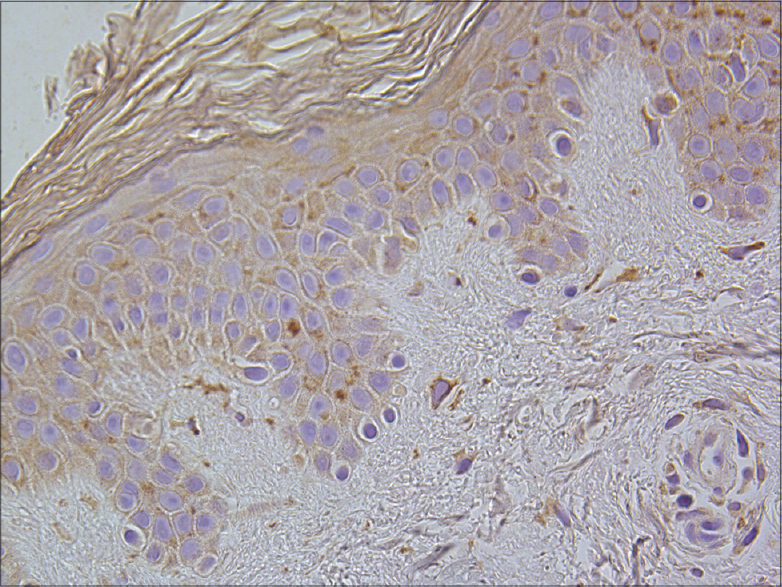 |
| Figure 10: A few to a moderate number of IL-8-containing keratinocytes and dermal connective tissue cells in healthy skin (IL-8 IHC, ×400) |
We found a strong positive correlation between HBD-2 and MMP-2 expression (Spearman's rank correlation coefficient was 0.886 [P < 0.001]), a moderate positive correlation between TNF-alpha and MMP-2 (Spearman's rank correlation coefficient was 0.795 [P < 0.001]) and a moderate positive correlation between IL-6 and IL-8 (Spearman's rank correlation coefficient 0.594 [P < 0.001]). The correlations between HBD-2 and TNF-alpha, TNF-alpha and IL-6 and TNF-alpha and IL-8 were weak. In contrast, we did not detect a correlation between HBD-2 and IL-6, HBD-2 and IL-8, MMP-2 and IL-6 or MMP-2 and IL-8.
We found statistically significant correlations between the following factors and disease severity:
- Moderate positive correlation between HBD-2 and disease severity; Spearman's rank correlation coefficient was 0.484 (P value 0.002)
- Weak positive correlation between MMP-2 and disease severity; Spearman's rank correlation coefficient was 0.318 (P value 0.046)
- Weak positive correlation between TNF-alpha and disease severity; Spearman's rank correlation coefficient was 0.363 (P value 0.021)
- Moderate negative correlation between IL-8 and disease severity; Spearman's rank correlation coefficient was 0.515 (P value 0.001).
No statistically significant correlations were found with gender or age of the psoriasis patient.
Discussion
Various types of human cells, including epithelial cells, express antimicrobial peptides. The peptides act on a broad spectrum of bacteria, fungi and viruses. They can disrupt or penetrate the bacterial membrane and interfere with intracellular functions. Human beta defensins link cell surface receptors to facilitate chemotaxis and in particular, HBD-2 can recruit immature dendritic cells and T cells to sites of interest. HBD-2 was previously shown to be produced by human epithelial cells in psoriatic skin.[14],[15] It is expressed at low levels in healthy keratinocytes and is often upregulated during inflammation or infection, suggesting that the HBD-2 increase is a secondary response if the primary defense against inflammation is not sufficient.[16] Previous studies have found a substantial relationship between an increase in human beta defensin genomic copy number and the risk of psoriasis onset.[17],[18] In our study, we evaluated HBD-2 expression in untreated psoriatic lesions and found that it was expressed in all samples. Furthermore, it was most highly expressed in inflammatory areas, consistent with the model of participation of the innate immune system in psoriasis. Some biopsies showed a significant decrease in HBD-2 expression. This finding may be related to the severity or length of the disease as fewer HBD-2 positive cells were found more commonly in patients with a history of psoriasis for only a few months. Patients with a longer course of psoriasis and more pronounced inflammation (both clinically and histologically) in their skin biopsy samples presented more obvious HBD-2 expression. Various studies have looked at serum levels of antimicrobial peptides as a possible marker of psoriasis severity or disease activity. Recent data suggest that HBD-2 serum levels in untreated patients may not relate to the severity of psoriasis but, their levels significantly increase after fumaric acid ester therapy. Additionally, treatment with the adipokine, visfatin can increase HBD-2 suggesting that psoriasis is related to metabolic syndromes.[19],[20] A decrease in HBD-2 serum levels has been detected after topical calcipotriol and betamethasone dipropionate combination therapy.[21] These data suggest that HBD-2 levels could be used as a possible marker for therapeutic efficacy.
Matrix metalloproteinases in serum and synovial fluid have become valuable biomarkers for the evaluation of psoriatic arthritis and its treatment with TNF inhibitors, which are also used in psoriasis.[22],[23] There are also available data that suggest that MMPs are important biomarkers of psoriasis activity and therapeutic response.[24],[25],[26],[27] Previous studies have found that ultraviolet radiation and vitamin D levels contribute to the severity, course and treatment success of psoriasis. It has been shown that serum vitamin D levels positively correlated with HBD-2 expression levels. In addition, a positive correlation exists between ultraviolet radiation and the expression of gelatinase MMP-2.[28],[29] We found a clear upregulation of MMP-2 expression in our samples; these data support the possibility that it could be utilized as a marker for psoriasis activity or a therapeutic target.[30] We found a strong positive correlation between MMP-2 and HBD-2 and both of them could be used as diagnostic/prognostic markers for psoriasis. In some of the psoriasis patients observed in this study, HBD-2 expression was low even though we could still find numerous MMP-2-positive cells. One potential explanation is that recent ultraviolet irradiation resulted in MMP upregulation. While antimicrobial peptide levels would be altered in such a situation, we excluded recently tanned patients from our study. Furthermore, we did not measure vitamin D levels in our patients and vitamin D could also be a possible key factor in HBD-2 and MMP-2 expression. We were unable to find any previous reports that simultaneously compared HBD-2 and MMP-2 in untreated psoriatic skin. Interestingly, the excessive expression of MMP-2 and almost absent HBD-2 expression has been found in biopsies of hidradenitis suppurativa, possibly due to MMP-2 providing a proteolytic environment and inactivating HBD-2.[31] MMP-2 also positively correlates with TNF-alpha, supporting the model that MMP-2 plays an important role in the pathogenesis of psoriasis.
The pro-inflammatory cytokines TNF-alpha, IL-6 and IL-8 are highly expressed in psoriasis and form a complex that induces the inflammatory signaling cascade resulting in the classic psoriatic plaque. Innate immune cells produce the key cytokines TNF-alpha and IL-6 which in turn activate myeloid dendritic cells which present antigens to produce new inflammatory mediators. Thus, T cells differentiate, keratinocytes are activated and antimicrobial peptide (HBD-2) and pro-inflammatory cytokines (TNF-alpha and IL6) are produced. IL-8 is a known cytokine with pro-inflammatory and growth-promoting activities and also induces keratinocyte proliferation.[1],[32],[33] HBD-2 can also induce cytokine production, such as TNF-alpha and IL-6.[34],[35] We detected variable, predominantly moderate expression of TNF-alpha, IL-6 and IL-8 in our skin samples with more prominent upregulation in the skin from patients with a long history of the disease. IL-6 and IL-8 showed a relevant positive correlation which is consistent with the current understanding of psoriasis pathogenesis. It appears that persistent skin inflammation is characterized by pro-inflammatory cytokine expression. Thus, these factors may become promising prognostic tools of the disease course.
Our data confirm that inflammatory cytokines are primary players in the pathogenesis of psoriasis but the antimicrobial peptide HBD-2 and gelatinase MMP-2 are also important factors and could be further considered in therapeutic approaches to psoriasis.
As a limitation, it should be noted that the study had a rather small patient number.
Conclusions
TNF-alpha, IL-6 and IL-8 are common cytokines expressed in psoriatic skin plaques to maintain the inflammatory cycle.
Antimicrobial peptide HBD-2, tissue degeneration marker MMP-2 and TNF-alpha positively correlate with the severity of psoriasis. On the other hand, the expression of IL-8 significantly decreases with clinically more severe psoriasis. Perhaps, HBD-2, MMP-2, TNF-alpha and IL-8 therefore can be considered as prognostic factors for psoriatic inflammation.
Financial support and sponsorship
”Support for doctoral students in mastering the study program and acquisition of a scientific degree in Rīga Stradiņš University,” agreement no: 2009/0147/1DP/1.1.2.1.2/09/IPIA/VIAA/009.
Conflicts of interest
There are no conflicts of interest.
| 1. |
Nestle FO, Kaplan DH, Barker J. Psoriasis. N Engl J Med 2009;361:496-509.
[Google Scholar]
|
| 2. |
Morizane S, Gallo RL. Antimicrobial peptides in the pathogenesis of psoriasis. J Dermatol 2012;39:225-30.
[Google Scholar]
|
| 3. |
Yamaguchi Y, Ouchi Y. Antimicrobial peptide defensin: identification of novel isoforms and the characterization of their physiological roles and their significance in the pathogenesis of diseases. Proc Jpn Acad Ser B Phys Biol Sci 2012;88:152-66.
[Google Scholar]
|
| 4. |
Borkowski AW, Gallo RL. The coordinated response of the physical and antimicrobial peptide barriers of the skin. J Invest Dermatol 2011;131:285-7.
[Google Scholar]
|
| 5. |
Schröder JM, Harder J. Human beta-defensin-2. Int J Biochem Cell Biol 1999;31:645-51.
[Google Scholar]
|
| 6. |
Fleischmajer R, Kuroda K, Hazan R, Gordon RE, Lebwohl MG, Sapadin AN, et al. Basement membrane alterations in psoriasis are accompanied by epidermal overexpression of MMP-2 and its inhibitor TIMP-2. J Invest Dermatol 2000;115:771-7.
[Google Scholar]
|
| 7. |
Baran W, Szepietowski JC, Mazur G, Baran E. IL-6 and IL-10 promoter gene polymorphisms in psoriasis vulgaris. Acta Derm Venereol 2008;88:113-6.
[Google Scholar]
|
| 8. |
Qazi BS, Tang K, Qazi A. Recent advances in underlying pathologies provide insight into interleukin-8 expression-mediated inflammation and angiogenesis. Int J Inflam 2011;2011:908468.
[Google Scholar]
|
| 9. |
Stefanini M, De Martino C, Zamboni L. Fixation of ejaculated spermatozoa for electron microscopy. Nature 1967;216:173-4.
[Google Scholar]
|
| 10. |
Fischer AH, Jacobson KA, Rose J, Zeller R. Hematoxylin and eosin staining of tissue and cell sections. CSH Protoc 2008;2008:pdb.prot4986.
[Google Scholar]
|
| 11. |
Hsu SM, Raine L, Fanger H. The use of antiavidin antibody and avidin-biotin-peroxidase complex in immunoperoxidase technics. Am J Clin Pathol 1981;75:816-21.
[Google Scholar]
|
| 12. |
Pilmane M, Luts A, Sundler F. Changes in neuroendocrine elements in bronchial mucosa in chronic lung disease in adults. Thora×1995;50:551-4.
[Google Scholar]
|
| 13. |
Christensen R. Analysis of Variance, Design and Regression: Applied Statistical Methods. London: Chapman and Hall; 1996.
[Google Scholar]
|
| 14. |
Kim JE, Kim BJ, Jeong MS, Seo SJ, Kim MN, Hong CK, et al. Expression and modulation of LL-37 in normal human keratinocytes, HaCaT cells, and inflammatory skin diseases. J Korean Med Sci 2005;20:649-54.
[Google Scholar]
|
| 15. |
Weinberg A, Jin G, Sieg S, McCormick TS. The yin and yang of human Beta-defensins in health and disease. Front Immunol 2012;3:294.
[Google Scholar]
|
| 16. |
Gallo RL, Nakatsuji T. Microbial symbiosis with the innate immune defense system of the skin. J Invest Dermatol 2011;131:1974-80.
[Google Scholar]
|
| 17. |
Hollox EJ, Huffmeier U, Zeeuwen PL, Palla R, Lascorz J, Rodijk-Olthuis D, et al. Psoriasis is associated with increased beta-defensin genomic copy number. Nat Genet 2008;40:23-5.
[Google Scholar]
|
| 18. |
Stuart PE, Hüffmeier U, Nair RP, Palla R, Tejasvi T, Schalkwijk J, et al. Association of ß-defensin copy number and psoriasis in three cohorts of European origin. J Invest Dermatol 2012;132:2407-13.
[Google Scholar]
|
| 19. |
Gambichler T, Bechara FG, Scola N, Rotterdam S, Altmeyer P, Skrygan M. Serum levels of antimicrobial peptides and proteins do not correlate with psoriasis severity and are increased after treatment with fumaric acid esters. Arch Dermatol Res 2012;304:471-4.
[Google Scholar]
|
| 20. |
Hau CS, Kanda N, Noda S, Tatsuta A, Kamata M, Shibata S, et al. Visfatin enhances the production of cathelicidin antimicrobial peptide, human ß-defensin-2, human ß-defensin-3, and S100A7 in human keratinocytes and their orthologs in murine imiquimod-induced psoriatic skin. Am J Pathol 2013;182:1705-17.
[Google Scholar]
|
| 21. |
Abdelmaksood R, Hashad D. The impact of topical calcipotriol and betamethasone on human beta-defensin 2 expression and serum level in psoriatic patients. Clin Lab 2013;59:277-82.
[Google Scholar]
|
| 22. |
Chandran V, Shen H, Pollock RA, Pellett FJ, Carty A, Cook RJ, et al. Soluble biomarkers associated with response to treatment with tumor necrosis factor inhibitors in psoriatic arthritis. J Rheumatol 2013;40:866-71.
[Google Scholar]
|
| 23. |
Giannelli G, Erriquez R, Iannone F, Marinosci F, Lapadula G, Antonaci S. MMP-2, MMP-9, TIMP-1 and TIMP-2 levels in patients with rheumatoid arthritis and psoriatic arthritis. Clin Exp Rheumatol 2004;22:335-8.
[Google Scholar]
|
| 24. |
Buommino E, De Filippis A, Gaudiello F, Balato A, Balato N, Tufano MA, et al. Modification of osteopontin and MMP-9 levels in patients with psoriasis on anti-TNF-α therapy. Arch Dermatol Res 2012;304:481-5.
[Google Scholar]
|
| 25. |
Flisiak I, Zaniewski P, Chodynicka B. Plasma TGF-beta1, TIMP-1, MMP-1 and IL-18 as a combined biomarker of psoriasis activity. Biomarkers 2008;13:549-56.
[Google Scholar]
|
| 26. |
Lee SE, Lew W. The Increased Expression of Matrix Metalloproteinase-9 Messenger RNA in the Non-lesional Skin of Patients with Large Plaque Psoriasis Vulgaris. Ann Dermatol 2009;21:27-34.
[Google Scholar]
|
| 27. |
Suomela S, Kariniemi AL, Snellman E, Saarialho-Kere U. Metalloelastase (MMP-12) and 92-kDa gelatinase (MMP-9) as well as their inhibitors, TIMP-1 and -3, are expressed in psoriatic lesions. Exp Dermatol 2001;10:175-83.
[Google Scholar]
|
| 28. |
Kim SK, Park S, Lee ES. Toll-like receptors and antimicrobial peptides expressions of psoriasis: correlation with serum vitamin D level. J Korean Med Sci 2010;25:1506-12.
[Google Scholar]
|
| 29. |
Koivukangas V, Kallioinen M, Autio-Harmainen H, Oikarinen A. UV irradiation induces the expression of gelatinases in human skin in vivo. Acta Derm Venereol 1994;74:279-82.
[Google Scholar]
|
| 30. |
Vasku V, Bienertova Vasku J, Slonková V, Kanková K, Vasku A. Matrix metalloproteinase-2 promoter variability in psoriasis. Arch Dermatol Res 2009;301:467-73.
[Google Scholar]
|
| 31. |
Mozeika E, Pilmane M, Nürnberg BM, Jemec GB. Tumour necrosis factor-alpha and matrix metalloproteinase-2 are expressed strongly in hidradenitis suppurativa. Acta Derm Venereol 2013;93:301-4.
[Google Scholar]
|
| 32. |
Barker CL, McHale MT, Gillies AK, Waller J, Pearce DM, Osborne J, et al. The development and characterization of an in vitro model of psoriasis. J Invest Dermatol 2004;123:892-901.
[Google Scholar]
|
| 33. |
Kapp A. The role of cytokines in the psoriatic inflammation. J Dermatol Sci 1993;5:133-42.
[Google Scholar]
|
| 34. |
Kanda N, Kamata M, Tada Y, Ishikawa T, Sato S, Watanabe S. Human ß-defensin-2 enhances IFN-γ and IL-10 production and suppresses IL-17 production in T cells. J Leukoc Biol 2011;89:935-44.
[Google Scholar]
|
| 35. |
Zalewska A, Glowacka E, Wyczólkowska J, Tchórzewski H, Narbutt J, Sysa-Jedrzejowska A. Interleukin 6 and 8 levels in plasma and fibroblast cultures in psoriasis. Mediators Inflamm 2006;2006:81767.
[Google Scholar]
|
Fulltext Views
5,059
PDF downloads
3,654





