Translate this page into:
Malignancy in dermatomyositis: A Bayesian Belief Network approach
Correspondence Address:
Bell Raj Eapen
Kaya Skin Clinic, Dubai
United Arab Emirates
| How to cite this article: Eapen BR. Malignancy in dermatomyositis: A Bayesian Belief Network approach. Indian J Dermatol Venereol Leprol 2007;73:445 |
Abstract
Background: Dermatomyositis (DMS) is an inflammatory disease affecting the muscle and the skin and is known to be associated with a definite risk of malignancy. Extensive malignancy screening may not be cost-effective in all patients. Several predictive factors have been postulated in DMS. Aims: This study attempts to build a Bayesian Belief Network model based on data available from literature to assign a numerical risk to each patient by taking the predictive factors into consideration. Methods: Relevant frequency data was collected from literature reports of dermatomyositis cases for four independent factors: age over 40, male sex, elevated erythrocyte sedimentation rate (ESR) and cutaneous necrosis. The model had 'malignancy risk' as the single decision variable. All evidence nodes had only two outcomes. The model was constructed using the GeNIe modeling environment and the user interface was implemented using VisualBasic.NET. Results: Four studies provided data for 151 DMS patients out of which 44 patients had malignancy. The constructed model had one decision node and four evidence nodes. The software to calculate the numerical risk is available for download from http://www.gulfdoctor.net/derm/dmbbn.htm. Conclusion: Bayesian Belief networks (BBN) can be used in situations like this where predictive factors are clearly associated with uncertainty. However, the present model may still be inaccurate because of the lack of reliable data and extensive testing.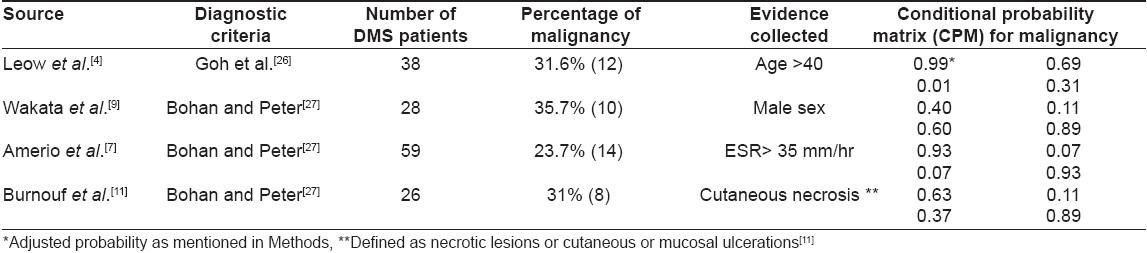

 |
| Figure 1: The Bayesian model with one decision node and four evidence nodes |
 |
| Figure 1: The Bayesian model with one decision node and four evidence nodes |
Introduction
Dermatomyositis (DMS) is a rare inflammatory disease with cutaneous and muscle manifestations. Despite a few reports to the contrary, dermatomyositis is widely accepted as a definite risk factor for internal malignancy with rates varying from 10 to 50%. [1]
The type of malignancy also varies considerably with sex and geographic location with ovarian and breast carcinoma predominating in women and lung cancer in men. [2] However, Asian studies have reported nasopharyngeal carcinoma as the most common type of malignancy associated with DMS. [3],[4] Thus, with the reports of varied types of carcinoma associated with DMS, malignancy screening in DMS can be time-consuming and expensive.
For cost-effectiveness, it might be important to develop a system to assess the potential risk of developing malignancy in individual patients. Several predictive factors have been described over the years with the age of the patient being the most important. [5] However, studies have shown that age should not be the only consideration in risk assessment. [6] Several other factors including male sex, [3] ESR, [7] skin lesions, [8] muscle biopsy findings [9] and enzyme levels [6] have been suggested as probable predictive factors. However, none of the factors consistently appeared in these studies.
In this scenario where different factors contribute an uncertain risk to the development of malignancy, Bayesian Belief Networks (BBN) could provide an effective method of knowledge representation and risk calculation thereby helping clinicians to decide whether to adopt an aggressive cancer screening procedure.
BBN is a graphical structure with "nodes" to represent variables and directed arcs (or links) representing relations among them. [10] The simplest form of BBN with a single "decision node" representing the malignancy risk and multiple "evidence nodes" representing predictive factors is sufficient for our purpose. Conditional probability matrices defining the probability of finding a factor given the outcome were extracted from literature references to build a BBN.
Using the BBN involves entering the "evidence" for a particular patient to assess the malignancy. A software was built in VB.NET for this purpose and was made freely available.
Methods
A literature search was done using Pubmed for articles on malignancy in dermatomyositis during the last 15 years from 1991 to 2006. Case reports, studies not discriminating polymyositis (PM) from dermatomyositis and studies of any single type of malignancy were excluded. The probable predictive factors identified were age, [4] sex, [9] increased ESR, [7] cutaneous necrosis, [11] muscle enzymes, [3] interstitial lung diseases, [3] amyopathic dermatomyositis, [12] muscle biopsy findings, [9] constitutional symptoms, [6] rapid onset of disease [6] and Raynaud′s phenomena. [6]
BBN makes the assumption that conditional probabilities are independent of each other. [13] However, the variables corresponding to the various muscle enzymes could be closely interrelated and correlated with the presence of cutaneous necrosis. [11] Hence, muscle enzymes were excluded from the model. Interstitial lung disease could be an independent risk factor for malignancy even without PM-DMS. [14] Although amyopathic dermatomyositis (ADM) showed an increased association with malignancy, [12] it cannot be considered independent from the other factors. Additionally, although "targetoid fiber" as a histopathological finding showed an association with malignancy, [9] the results were inconclusive. Hence, these factors were also excluded from the model. Though constitutional symptoms, rapid onset of myositis and Raynaud′s phenomenon were identified as significant factors in a single study, [6] the available data did not discriminate between PM and DMS.
A prior probability has to be assigned to the only decision node, ′Malignancy Risk′. [13] This was calculated by dividing the total number of patients with malignancy in the studies contributing evidence by the total number of patients included in these studies.
BBN requires data in the form of a "conditional probability matrix" (CPM). [15] To define the CPM for each factor, the percentages of patients with the factor in the malignancy group and the no-Malignancy group must be available. Fortunately that data was available for the remaining predictive factors-age, sex, ESR and cutaneous necrosis.
In the study which provided evidence for age, [4] all the patients with malignancy (although this was a small sample size) were over the age of 40. This evaluates to a probability of 100% for ages over 40. A probability of 100% is extremely rare in clinical situations and a probability model may behave unexpectedly if 100% probability is assigned. Hence, probability for this factor was adjusted to 99%.
The model was constructed using the GeNIe modeling environment developed by the Decision Systems Laboratory of the University of Pittsburgh (http://dsl.sis.pitt.edu). The user interface was implemented using VisualBasic.NET.
Results
Four studies provided the data for conditional probability matrix [Table - 1]. A total of 151 DMS patients were present in these studies out of which 44 patients had malignancy. Hence, the prior probability of the decision variable was set at 0.29 (29%). The conditional probabilities of the four evidence variables (age > 40, male sex, ESR > 35 mm/h and cutaneous necrosis) derived from the relevant studies are listed in [Table - 1]. All evidence variables had only two probable values (present or absent). The constructed model is shown in [Figure - 1]. The implementation is available for download from http://www.gulfdoctor.net/derm/dmbbn.htm.
Discussion
Although the paraneoplastic nature of a subset of dermatomyositis cases is clearly demonstrated, its association with several disparate malignancies makes extensive malignancy screening difficult and expensive. [6],[16] Several predictive factors were identified, however, no factor consistently showed significance in multivariate analysis. [3] This may be due to limitations in sample size as dermatomyositis is a rare condition. However, a malignancy risk assessment is important in the management of DMS patients. As the importance of each predictive factor in the causation of malignancy is clearly associated with uncertainty, a BBN may be useful in assigning a numerical risk to the patient with reasonable accuracy.
In BBN, uncertainty is represented by conditional probabilities which express the likelihood of the predictive factor being present if malignancy is present or absent in the patient. [17] For this, the assumption has to be made that the conditional probabilities are independent of each other. [13] Conditional probabilities can be derived from actual frequency data, published data or personal experience. In this study, probabilities are derived from published frequency data.
Conditional probability matrix construction is the most crucial step in a BBN model. Relevant frequency data was available for four factors as mentioned in the previous section. The constructed BBN model had only four evidence nodes and one decision node and was simple as the evidence had only two probable values. A BBN is more useful if a user interface is available to enter the evidence and to compute the risk for a given patient. [18] Such an interface was constructed and made freely available.
Bayesian Belief Network algorithm has been extensively researched by Pearl. [19] Bayesian Belief networks have been successfully used in several other areas. [20],[21],[22],[23] BBN has successfully been used in dermatology mainly for melanoma studies. [24],[25]
The main drawback with the current study is that only four factors could be included in the model though several other predictive factors were suspected. Only one study per factor was available for data collection. The sample size was limited in the selected studies. A BBN should ideally be tested based on patient data with the model being further improved based on performance. [17] However, this was not performed because of the lack of a reliable series of patient data.
This clinically significant study demonstrates the usefulness of BBN in the assessment of malignancy risk in DMS. However, the model needs further refinement using more data, proper testing and input from experts.
Acknowledgment
The model described in this paper was created using the GeNIe modeling environment and the implementation is based on the SMILE reasoning engine. Both are developed by the Decision Systems Laboratory of the University of Pittsburgh (http://dsl.sis.pitt.edu) and are freely available for download from their website.[27]
| 1. |
Ghate J, Katsambas A, Augerinou G, Jorizzo JL. Review article: A therapeutic update on dermatomyositis/polymyositis. Int J Dermatol 2000;39:81-7.
[Google Scholar]
|
| 2. |
Airio A, Pukkala E, Isomaki H. Elevated cancer incidence in patients with dermatomyositis: A population based study. J Rheumatol 1995;22:1300-3.
[Google Scholar]
|
| 3. |
Chen YJ, Wu CY, Shen JL. Predicting factors of malignancy in dermatomyositis and polymyositis: A case-control study. Br J Dermatol 2001;144:825-31.
[Google Scholar]
|
| 4. |
Leow YH, Goh CL. Malignancy in adult dermatomyositis. Int J Dermatol 1997;36:904-7.
[Google Scholar]
|
| 5. |
Basset-Seguin N, Roujeau JC, Gherardi R, Guillaume JC, Revuz J, Touraine R. Prognostic factors and predictive signs of malignancy in adult dermatomyositis. A study of 32 cases. Arch Dermatol 1990;126:633-7.
[Google Scholar]
|
| 6. |
Sparsa A, Liozon E, Herrmann F, Ly K, Lebrun V, Soria P, et al . Routine vs extensive malignancy search for adult dermatomyositis and polymyositis: A study of 40 patients. Arch Dermatol 2002;138:885-90.
[Google Scholar]
|
| 7. |
Amerio P, Girardelli CR, Proietto G, Forleo P, Cerritelli L, Feliciani C, et al . Usefulness of erythrocyte sedimentation rate as tumor marker in cancer associated dermatomyositis. Eur J Dermatol 2002;12:165-9.
[Google Scholar]
|
| 8. |
Gallais V, Crickx B, Belaich S. Prognostic factors and predictive signs of malignancy in adult dermatomyositis. Ann Dermatol Venereol 1996;123:722-6.
[Google Scholar]
|
| 9. |
Wakata N, Kurihara T, Saito E, Kinoshita M. Polymyositis and dermatomyositis associated with malignancy: A 30-year retrospective study. Int J Dermatol 2002;41:729-34.
[Google Scholar]
|
| 10. |
Korb K, Nicholson A. Bayesian artificial intelligence. Chapman and Hall/CRC: London; 2004.
[Google Scholar]
|
| 11. |
Burnouf M, Mahe E, Verpillat P, Descamps V, Lebrun-Vignes B, Picard-Dahan C, et al . Cutaneous necrosis is predictive of cancer in adult dermatomyositis. Ann Dermatol Venereol 2003;130:313-6.
[Google Scholar]
|
| 12. |
Whitmore SE, Watson R, Rosenshein NB, Provost TT. Dermatomyositis sine myositis: Association with malignancy. J Rheumatol 1996;23:101-5.
[Google Scholar]
|
| 13. |
Montironi R, Whimster WF, Collan Y, Hamilton PW, Thompson D, Bartels PH. How to develop and use a Bayesian Belief Network. J Clin Pathol 1996;49:194-201.
[Google Scholar]
|
| 14. |
Douglas WW, Tazelaar HD, Hartman TE, Hartman RP, Decker PA, Schroeder DR, et al . Polymyositis-dermatomyositis-associated interstitial lung disease. Am J Respir Crit Care Med 2001;164:1182-5.
[Google Scholar]
|
| 15. |
Kazi JI, Furness PN, Nicholson M. Diagnosis of early acute renal allograft rejection by evaluation of multiple histological features using a Bayesian belief network. J Clin Pathol 1998;51:108-13.
[Google Scholar]
|
| 16. |
Hill CL, Zhang Y, Sigurgeirsson B, Pukkala E, Mellemkjaer L, Airio A, et al . Frequency of specific cancer types in dermatomyositis and polymyositis: A population-based study. Lancet 2001;357:96-100.
[Google Scholar]
|
| 17. |
Hamilton PW, Anderson N, Bartels PH, Thompson D. Expert system support using Bayesian belief networks in the diagnosis of fine needle aspiration biopsy specimens of the breast. J Clin Pathol 1994;47:329-36.
[Google Scholar]
|
| 18. |
Morrison ML, McCluggage WG, Price GJ, Diamond J, Sheeran MR, Mulholland KM, et al . Expert system support using a Bayesian belief network for the classification of endometrial hyperplasia. J Pathol 2002;197:403-14.
[Google Scholar]
|
| 19. |
Pearl J. Probabilistic reasoning in intelligent systems. San Diego, California: Morgan Kaufman Publishing; 1988.
[Google Scholar]
|
| 20. |
Antal P, Fannes G, Timmerman D, Moreau Y, De Moor B. Bayesian applications of belief networks and multilayer perceptrons for ovarian tumor classification with rejection. Artif Intell Med 2003;29:39-60.
[Google Scholar]
|
| 21. |
Mazzucchelli R, Santinelli A, Colanzi P, Streccioni M, Lopez-Beltran A, Scarpelli M, et al . Urothelial papillary lesions. Development of a Bayesian Belief Network for diagnosis and grading. Anticancer Res 2001;21:1157-62.
[Google Scholar]
|
| 22. |
Nikiforidis GC, Sakellaropoulos GC. Expert system support using Bayesian belief networks in the prognosis of head-injured patients of the ICU. Med Inform (Lond) 1998;23:1-18.
[Google Scholar]
|
| 23. |
Montironi R, Diamanti L, Pomante R, Thompson D, Bartels PH. Subtle changes in benign tissue adjacent to prostate neoplasia detected with a Bayesian belief network. J Pathol 1997;182:442-9.
[Google Scholar]
|
| 24. |
Do KA, Aitken JF, Green AC, Martin NG. Analysis of melanoma onset: Assessing familial aggregation by using estimating equations and fitting variance components via Bayesian random effects models. Twin Res 2004;7:98-113.
[Google Scholar]
|
| 25. |
Sierra B, Larranaga P. Predicting survival in malignant skin melanoma using Bayesian networks automatically induced by genetic algorithms. An empirical comparison between different approaches. Artif Intell Med 1998;14:215-30.
[Google Scholar]
|
| 26. |
Goh CL, Rajan VS. Dermatomyositis in a skin clinic. Ann Acad Med Singapore 1983;12:6-12.
[Google Scholar]
|
| 27. |
Bohan A, Peter JB. Polymyositis and dermatomyositis (first of two parts). N Engl J Med 1975;292:344-7.
[Google Scholar]
|
Fulltext Views
1,502
PDF downloads
1,927





