Translate this page into:
Metabolic syndrome in androgenic alopecia
Correspondence Address:
Gatha M Upadya
Department of Dermatology, Kasturba Medical College Hospital, Attavar, Mangalore - 575 001, Karnataka
India
| How to cite this article: Gopinath H, Upadya GM. Metabolic syndrome in androgenic alopecia. Indian J Dermatol Venereol Leprol 2016;82:404-408 |
Abstract
Background: Androgenic alopecia has been associated with an increased risk of coronary heart disease in various studies. The relationship between androgenic alopecia and metabolic syndrome, a known risk factor for atherosclerotic cardiovascular disease, is still poorly understood. Aim: To study the association between metabolic syndrome and early-onset androgenic alopecia. Methods: A hospital-based analytical cross-sectional study was done on men in the age group of 18–55 years. Eighty five clinically diagnosed cases with early-onset (<35 years) androgenic alopecia of Norwood grade III or above, and 85 controls without androgenic alopecia were included. Data collected included anthropometric measurements, arterial blood pressure and history of chronic diseases. Fasting blood and lipid profile were determined. Metabolic syndrome was diagnosed as per the new International Diabetes Federation criteria. Chi-square and Student's t-test were used for statistical analysis using Statistical Package for the Social Sciences (SPSS) version 17.00. Results: Metabolic syndrome was seen in 19 (22.4%) patients with androgenic alopecia and 8 (9.4%) controls (P = 0.021). Abdominal obesity, hypertension and lowered high-density lipoprotein were significantly higher in patients with androgenic alopecia versus their respective controls. Limitations: The limitations of our study include small sample size in subgroups and the lack of evidence of a temporal relationship between metabolic syndrome and androgenic alopecia. Conclusion: A higher prevalence of metabolic syndrome is seen in men with early-onset androgenic alopecia. Early screening for metabolic syndrome and its components is beneficial in patients with early-onset androgenic alopecia.Introduction
Androgenic alopecia is the most common type of hair loss in men, characterized by transformation of thick terminal hair follicles into thin vellus-like hair follicles. It occurs under the influence of androgens in genetically predisposed individuals.[1] The mode of inheritance is likely to be polygenic.[2] Various studies have associated androgenic alopecia with increased risk of coronary heart disease, but the pathophysiology is poorly understood.[3],[4],[5] Metabolic syndrome is a cluster of inter-related risk factors that increase the risk of atherosclerotic cardiovascular disease.[6] Our study looked at the association between metabolic syndrome and early-onset androgenic alopecia in a southern Indian city.
Methods
We undertook a hospital-based analytical cross-sectional study of 85 cases and 85 age-matched controls attending the skin outpatient department between September 2011 and August 2013. Men in the age group 18–55 years with clinically diagnosed early-onset androgenic alopecia (age <35 years) with Norwood type III or above were included as cases. Men aged between 18 and 55 years with a dermatological disease other than androgenic alopecia were included as controls.[7] Most controls had warts or benign nevi. The study was limited to patients <55 years of age due to the increase in the prevalence of androgenic alopecia with age.[8] Patients receiving hormone replacement therapy with testosterone, those on corticosteroid therapy and those with hypothyroidism and psoriasis were excluded to minimize the role of other variables in the pathogenesis of androgenic alopecia and metabolic syndrome. The study was limited to men because of the possible differences in pathogenesis and the controversial role of androgens in female pattern hair loss.[9]
Permission to conduct the study was taken from the time-bound Ethics Committee of the Institution. After obtaining informed consent, detailed history and clinical examination were conducted and the details entered in a proforma. The history included age, occupation, duration of alopecia (based on patient history), treatment details for alopecia, smoking, alcohol consumption and sedentariness (<30 min of physical activity/day). Personal history of, and drug history for hypertension, diabetes mellitus and dyslipidemia was obtained. Diagnosis of androgenic alopecia was based on history and clinical findings including early age of onset (<35 years) and pattern of increased hair thinning on frontal and parietal scalp with greater hair density on the occipital scalp. Family history of androgenic alopecia was also noted. The degree of androgenic alopecia was based on the Norwood scale (III–VII). Data on weight, height and waist circumference was recorded. Waist circumference was measured at the midpoint between the lower margin of the last palpable rib and the top of the iliac crest at the end of a normal expiration. Systolic and diastolic blood pressure (BP) were measured after a 5-min rest and again 10 min later, recording the mean value. Body mass index was calculated by dividing weight (in kg) by height (in m2). Fasting plasma sugar and complete lipid profile were analyzed in blood samples drawn between 8 and 9 am, after a 12 h fast.
The metabolic syndrome was diagnosed according to the new International Diabetes Federation definition as: central obesity (defined as waist circumference with ethnicity-specific value ≥90 cm for Indian men) plus any two of the following four factors: (1) raised triglycerides ≥150 mg/dL (1.7 mmol/L) or specific treatment for this lipid abnormality.[10] (2) reduced high-density lipoprotein (HDL) cholesterol <40 mg/dL (1.03 mmol/L) in men and <50 mg/dL (1.29 mmol/L) in women or specific treatment for this lipid abnormality, (3) raised BP: Systolic BP ≥130 or diastolic BP ≥85 mm of Hg or treatment of previously diagnosed hypertension, (4) raised fasting plasma glucose (FPG) ≥100 mg/dL (5.6 mmol/L) or previously diagnosed type two diabetes.
Statistical analysis
Analysis was carried out using Statistical Package for the Social Sciences (SPSS) version 17.00 (SPSS Inc. 233, South Walker Drive, 11th Floor, Chicago, IL) The Student's t-test was applied to compare mean values of quantitative variables. Qualitative variables were analyzed with a Chi-square test. P < 0.05 was considered significant. The sample size of the population was determined by positing the prevalence of metabolic syndrome in the control group as 10% and estimated 3-fold increase in metabolic syndrome in patients with androgenic alopecia (based on earlier studies). For significance level (α) at 0.05 and power factor (1-β) at 80%, it was necessary to have at least 78 patients per group.
Results
The study included 85 cases and 85 controls. The descriptive characteristics of the study are given in [Table - 1].
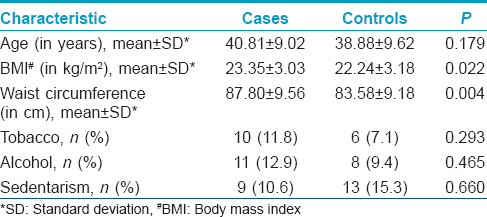
The mean duration of alopecia was 14.8 years. Family history of androgenic alopecia was significantly higher in 46 (54.1%) cases compared to 25 (29.4%) controls, (P = 0.002). Most (20%) patients had grade 5 alopecia. The distribution of patients according to the degree of alopecia is given in [Table - 2].

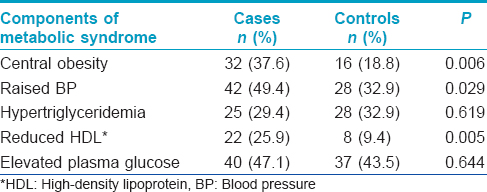
Metabolic syndrome was seen in 19 (22.4%) cases and 8 (9.4%) controls (P = 0.021). Prevalence of central obesity, hypertension and reduced HDL were significantly higher in cases compared to controls. Hypertension and elevated plasma glucose were the most common components of the metabolic syndrome noted in our patients. The distribution of components of metabolic syndrome is given in [Table - 3].
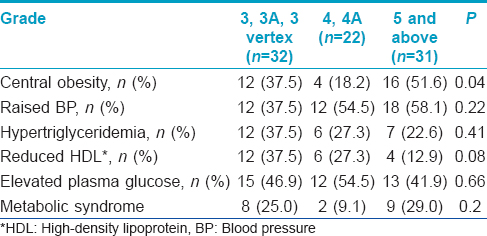
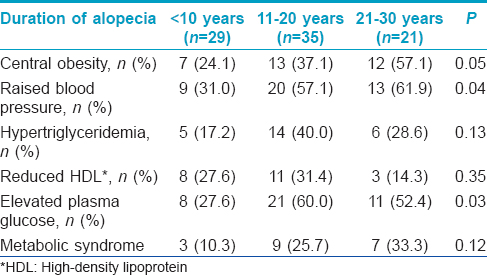
Though the sample size in the groups was small, central obesity was significantly associated with grade of alopecia [Table - 4]. Hypertension and elevated plasma glucose were significantly associated with increased duration of alopecia [Table - 5].
Discussion
Cardiovascular disease has become a leading cause of mortality in most countries.[11] Endogenous androgens have not shown any consistent causal association with coronary artery disease.[12] Metabolic syndrome has been associated with higher cardiovascular and coronary heart disease mortality even after adjusting for other cardiovascular disease risk factors. The risk increases with number of metabolic syndrome components present.[13]
A few studies have been done on the association of metabolic syndrome with androgenic alopecia. Our study showed an increased prevalence of metabolic syndrome in patients with early-onset androgenic alopecia. Arias-Santiago et al. in a case–control study of 77 participants with early-onset androgenic alopecia and 77 controls demonstrated metabolic syndrome in 60% of men with androgenic alopecia, 48.6% of women with androgenic alopecia, 12.5% of male controls and 8.1% of female controls (P < 0.0001).[14] The lower incidence of sedentarism might have resulted in the lower incidence of metabolic syndrome in our study. A pro-inflammatory state has been suggested as a possible link between the pathogenesis of androgenic alopecia and metabolic syndrome.
Su and Chen conducted a population-based cross-sectional survey of 740 participants and found a statistically significant association between androgenic alopecia and the presence of metabolic syndrome as well as between androgenic alopecia and components of metabolic syndrome after controlling for confounding factors.[15]
Our study did not find any significant association between elevated plasma glucose and androgenic alopecia. Insulin resistance has been proposed as the underlying pathophysiological cause of metabolic syndrome[16] but this is not adequately reflected by fasting plasma glucose, the measure we used. Matilainen et al. found a 2-fold risk for hyperinsulinemia in men with early-onset androgenic alopecia compared with controls.[17] Insulin and insulin resistance parameters were not evaluated in our study. Similar to our study, Ekmekci et al. did not find a significant difference in fasting blood glucose in women with androgenic alopecia and controls.[18] However, they found significantly higher HOMA insulin resistance and fasting insulin resistance index in the androgenic alopecia group.
Hypertension was significantly associated with androgenic alopecia compared to controls in our study. Ahouansou et al. found a strong association between hypertension and androgenic alopecia in 250 Caucasian men.[19] They proposed that androgens which bind to mineralocorticoid receptors cause elevated blood pressure or hyperaldosteronism which is associated with most primary hypertensions and may play a role in the pathogenesis of alopecia. Arias-Santiago et al. also demonstrated significantly elevated aldosterone levels and hypertension in women with early-onset androgenic alopecia compared to controls.[20]
Reduced levels of HDL cholesterol were significantly more common in men with androgenic alopecia compared to controls. Sadighha and Zahed demonstrated lower HDL cholesterol and higher triglyceride levels in men with vertex type androgenic alopecia than in controls.[21] Dyslipidemia may contribute to the risk of cardiovascular disease associated with androgenic alopecia.
We found significantly higher body mass index and waist circumference in patients with androgenic alopecia. Hirsso et al. also demonstrated higher body mass index and waist circumference in young men with moderate to extensive alopecia compared to men with little or no alopecia.[22] Waist circumference correlates with abdominal fat mass (subcutaneous and intra-abdominal). Increased waist circumference in addition to body mass index is a risk factor for mortality.[23]
Higher prevalence and earlier onset of coronary heart disease have been seen in South Asians probably due to genetic and environmental influences.[11] We were unable to find any previous studies of the association of metabolic syndrome and androgenic alopecia in a southern Indian population.
The limitations of our study include the small sample sizes in subgroups. Further studies with large sample sizes are needed to establish the relationship between the grade or duration of androgenic alopecia with metabolic syndrome, as well as its components. The duration of alopecia was based on patient history which might not be accurate particularly in a condition which has a gradual onset. Since our study was a cross-sectional study the temporal relationship between metabolic syndrome and androgenic alopecia could not be established.
Conclusion
An association between metabolic syndrome and early-onset androgenic alopecia was seen; this may contribute to the predisposition of patients with androgenic alopecia to develop cardiovascular disease. Early screening and interventions for metabolic syndrome and its components in patients with early-onset androgenic alopecia may prevent the development of cardiovascular disease.
Financial support and sponsorship
This study was partly supported by the research funds of Manipal University.
Conflicts of interest
There are no conflicts of interest.
| 1. |
Paus R, Olsen EA, Messenger AG. Hair growth disorders. In: Wolff K, Goldsmith LA, Katz SI, Gilchrest BA, Paller As, Leffell DJ, editors. Fitzpatrick's Dermatology in General Medicine. 7th ed. USA: The McGraw-Hill Companies Inc.; 2008. p. 753-77.
[Google Scholar]
|
| 2. |
Ellis JA, Stebbing M, Harrap SB. Genetic analysis of male pattern baldness and the 5alpha-reductase genes. J Invest Dermatol 1998;110:849-53.
[Google Scholar]
|
| 3. |
Cotton SG, Nixon JM, Carpenter RG, Evans DW. Factors discriminating men with coronary heart disease from healthy controls. Br Heart J 1972;34:458-64.
[Google Scholar]
|
| 4. |
Lesko SM, Rosenberg L, Shapiro S. A case-control study of baldness in relation to myocardial infarction in men. JAMA 1993;269:998-1003.
[Google Scholar]
|
| 5. |
Herrera CR, D'Agostino RB, Gerstman BB, Bosco LA, Belanger AJ. Baldness and coronary heart disease rates in men from the Framingham Study. Am J Epidemiol 1995;142:828-33.
[Google Scholar]
|
| 6. |
Grundy SM, Brewer HB Jr, Cleeman JI, Smith SC Jr, Lenfant C, American Heart Association, et al. Definition of metabolic syndrome: Report of the National Heart, Lung, and Blood Institute/American Heart Association conference on scientific issues related to definition. Circulation 2004;109:433-8.
[Google Scholar]
|
| 7. |
Norwood OT. Male pattern baldness: Classification and incidence. South Med J 1975;68:1359-65.
[Google Scholar]
|
| 8. |
Severi G, Sinclair R, Hopper JL, English DR, McCredie MR, Boyle P, et al. Androgenetic alopecia in men aged 40-69 years: Prevalence and risk factors. Br J Dermatol 2003;149:1207-13.
[Google Scholar]
|
| 9. |
Mubki T, Shamsaldeen O, McElwee KJ, Shapiro J. An update on diagnosis and treatment of female pattern hair loss. Expert Rev Dermatol 2013;8:427-36.
[Google Scholar]
|
| 10. |
Alberti KG, Zimmet P, Shaw J, IDF Epidemiology Task Force Consensus Group. The metabolic syndrome – A new worldwide definition. Lancet 2005;366:1059-62.
[Google Scholar]
|
| 11. |
Reddy KS, Yusuf S. Emerging epidemic of cardiovascular disease in developing countries. Circulation 1998;97:596-601.
[Google Scholar]
|
| 12. |
Wu FC, von Eckardstein A. Androgens and coronary artery disease. Endocr Rev 2003;24:183-217.
[Google Scholar]
|
| 13. |
Eberly LE, Prineas R, Cohen JD, Vazquez G, Zhi X, Neaton JD, et al. Metabolic syndrome: Risk factor distribution and 18-year mortality in the multiple risk factor intervention trial. Diabetes Care 2006;29:123-30.
[Google Scholar]
|
| 14. |
Arias-Santiago S, Gutiérrez-Salmerón MT, Castellote-Caballero L, BuendÍa-Eisman A, Naranjo-Sintes R. Androgenetic alopecia and cardiovascular risk factors in men and women: A comparative study. J Am Acad Dermatol 2010;63:420-9.
[Google Scholar]
|
| 15. |
Su LH, Chen TH. Association of androgenetic alopecia with metabolic syndrome in men: A community-based survey. Br J Dermatol 2010;163:371-7.
[Google Scholar]
|
| 16. |
Reaven GM. Banting lecture 1988. Role of insulin resistance in human disease. Diabetes 1988;37:1595-607.
[Google Scholar]
|
| 17. |
Matilainen V, Koskela P, Keinänen-Kiukaanniemi S. Early androgenetic alopecia as a marker of insulin resistance. Lancet 2000;356:1165-6.
[Google Scholar]
|
| 18. |
Ekmekci TR, Ucak S, Basat O, Koslu A, Altuntas Y. The presence of insulin resistance and comparison of various insulin sensivity indices in women with androgenetic alopecia. Eur J Dermatol 2007;17:21-5.
[Google Scholar]
|
| 19. |
Ahouansou S, Le Toumelin P, Crickx B, Descamps V. Association of androgenetic alopecia and hypertension. Eur J Dermatol 2007;17:220-2.
[Google Scholar]
|
| 20. |
Arias-Santiago S, Gutiérrez-Salmerón MT, BuendÍa-Eisman A, Girón-Prieto MS, Naranjo-Sintes R. Hypertension and aldosterone levels in women with early-onset androgenetic alopecia. Br J Dermatol 2010;162:786-9.
[Google Scholar]
|
| 21. |
Sadighha A, Zahed GM. Evaluation of lipid levels in androgenetic alopecia in comparison with control group. J Eur Acad Dermatol Venereol 2009;23:80-1.
[Google Scholar]
|
| 22. |
Hirsso P, Rajala U, Hiltunen L, Jokelainen J, Keinänen-Kiukaanniemi S, Näyhä S. Obesity and low-grade inflammation among young Finnish men with early-onset alopecia. Dermatology 2007;214:125-9.
[Google Scholar]
|
| 23. |
Koster A, Leitzmann MF, Schatzkin A, Mouw T, Adams KF, van Eijk JT, et al. Waist circumference and mortality. Am J Epidemiol 2008;167:1465-75.
[Google Scholar]
|
Fulltext Views
8,863
PDF downloads
3,027





