Translate this page into:
Methylprednisolone pulse therapy for leprosy neuritis: A retrospective study with sensory testing and peripheral nerve ultrasonography correlation
Corresponding author: Dr. Helena B. Lugão, Av. Bandeirantes, 3900, Campus Universitário, Ribeirão Preto, São Paulo, Brazil. helenalugao@hotmail.com
-
Received: ,
Accepted: ,
Read ERRATUM associated with this - 10.25259/IJDVL_1280_20_ER
How to cite this article: Lugão HB, Savarese LG, Silva SR, Barbosa MH, Foss NT, Frade MA. Methylprednisolone pulse therapy for leprosy neuritis: A retrospective study with sensory testing and peripheral nerve ultrasonography correlation. Indian J Dermatol Venereol Leprol 2022;88:114-6.
Sir,
Patients with leprosy neuritis are more likely to have disability. Nerve ultrasonography (USG) is inexpensive, widely available and can aid diagnosis and follow-up of neuritis.1-3 Oral steroids are the mainstay of treatment of neuritis. Pulsed intravenous corticosteroids may be used for severe neuritis, recalcitrant neuritis or for patients suffering from neuritis who have contraindications for oral steroids.4
We retrospectively reviewed data from 21 leprosy patients with neuritis, treated with pulse therapy at the National Reference Centre in Sanitary Dermatology Focusing on Leprosy of Ribeirão Preto Clinical Hospital – Brazil [Table 1]. Records of patients suffering from hypothyroidism, HIV infection, traumatic and hereditary neuropathies were not reviewed. Four diabetic patients were included in the study.
| Age | |
|---|---|
| Age range (years) | 12–63 |
| Mean±SD | 49.95±12.06 |
| Sex | n(%) |
| Male | 17 (81%) |
| Female | 4 (19%) |
| Leprosy classification | n(%) |
| Borderline-tuberculoid | 2 (9.5%) |
| Borderline-borderline | 4 (19%) |
| Borderline-lepromatous | 6 (28.6%) |
| Lepromatous | 4 (19%) |
| Primary neural leprosy | 5 (23.8%) |
| Slit skin smear | n(%) |
| Positive | 10 (47.6%) |
| Negative | 11 (52.4%) |
| Leprosy reaction* | n(%) |
| Isolated neuritis | 10 (47.6%) |
| Type 1 | 6 (28.6%) |
| Type 2 | 8 (38.1%) |
| Multidrug Therapy | n(%) |
| Pulses were done during MDT | 15 (71.4%) |
| Pulses were done after MDT | 6 (28.6%) |
| Anti-reaction treatment before pulse-therapy | |
| Pulse was initial anti-reaction treatment | 5 (23.8%) |
| Prednisone | 10 (47.6%) |
| Months of prednisone use before pulses: range (mean±SD) | 2–29 (10±8.25) |
| Prednisone + thalidomide | 6 (28.6%) |
| Months of prednisone use before pulses: range (mean±SD) | 17–72 (41±17.91) |
The patients had received intravenous methylprednisolone 1 g/day for three days in the first pulse and 1 g/day for one day in the subsequent pulses.4 The interval between pulses was approximately one month; all patients received at least one cycle, consisting of three pulses. Four patients needed another cycle 6–33 months after the first one due to recurrent neuritis; therefore, data from 25 cycles were analysed. The patients underwent skin and neurological examination monthly; prednisone and thalidomide doses were individually tailored according to clinical response.
Sensory testing using Semmes-Weinstein monofilaments (0.05 g, 0.2 g, 2 g, 4 g, 10 g and 300 g; 7 points on each hand and 10 points on each foot) was performed before and after each pulse therapy cycle. Poor sensory testing outcome was defined as significant worsening (≥2 monofilament grades) in more than 20% of hands or feet points.
All patients underwent peripheral nerve ultrasonography before and after each cycle of pulse therapy. The ulnar nerve (proximal to the cubital tunnel and at the cubital tunnel), median nerve and common fibular nerve were scanned along their transverse and longitudinal axes.3 Poor cross-sectional areas outcomes were defined elsewhere.3 Intraneural or epineural arterial blood flow pattern detected by color/ power Doppler (pulse repetition frequency 0.7–1 kHz) was considered indicative of nerve hypervascularity.
Statistical analysis included Wilcoxon, Fisher and McNemar tests and Spearman coefficients. We considered p value< 0.05 as statistically significant.
Adjuvant treatment given pre- and post-pulse is shown in Table 2. Most patients had their prednisone (14/16, 87.5%) and thalidomide (5/6, 83.3%) doses reduced after pulses. Eight patients discontinued prednisone use within six months. No patient had major adverse events.
| Prednisone (n=16) | Pre-pulse | Post-pulse* | |
|---|---|---|---|
| Minimum dose | 40 mg | zero | |
| Maximum dose | 80 mg | 30 mg | |
| Median | 60 mg | 10 mg | |
| Mean (mg/kg/day) | 0.76 | 0.17 | |
| Mean±SD | 58±12.4 mg | 13.1±10.8 mg | <0.0001† |
| Thalidomide (n=6) | Pre-pulse | Post-pulse* | |
| Minimum dose | 100 mg | zero | |
| Maximum dose | 200 mg | 200 mg | |
| Median | 200 mg | 100 mg | |
| Mean±SD | 183±40.8 mg | 100±63.3 mg | p=0.004† |
Table 3 shows sensory testing data. Only six patients (28.6%) had poor sensory outcomes. Patients with type 2 reactions had a higher frequency of poor sensory outcomes (5/11 pulse therapy cycles, 45.5%) compared to patients with isolated neuritis (3/11 cycles, 27.3%) and type 1 reactions (2/7 cycles, 28.6%) (p > 0.05 for all comparisons).
| Pre-pulse | Post-Pulse | P-value | |
|---|---|---|---|
| Abnormal sensory testing points* | |||
| Hands | 53.9% (187/347) | 47.7% (167/350) | >0.05 |
| Feet | 55.4% (276/498) | 48.1% (239/497) | >0.05 |
| Patients with grade 1 disability† | |||
| Hands | 14/21 | 13/21 | >0.05 |
| Feet | 17/21 | 15/21 | >0.05 |
There were no significant differences between pre- and post-pulse cross-sectional areas for any nerve. The frequencies of poor cross-sectional area outcomes were: 30/50 for ulnar nerve proximal to the cubital tunnel (60), 21/50 for ulnar nerve at cubital tunnel (42%), 23/50 for the median nerve (46%) and 29/48 for the common fibular nerve (60.4%). Figure 1 shows the frequencies of poor cross-sectional area outcomes in patients with isolated neuritis, type 1 and type 2 reactions. The frequency of nerves with positive Doppler signal decreased after pulses (65/198, 32.8% pre-pulse and 35/198 and 17.7% post-pulse; p = 0.0004). Pre-pulse positive Doppler signal was significantly associated with poor cross-sectional area outcome in ulnar nerves proximal to cubital tunnel and median nerves.
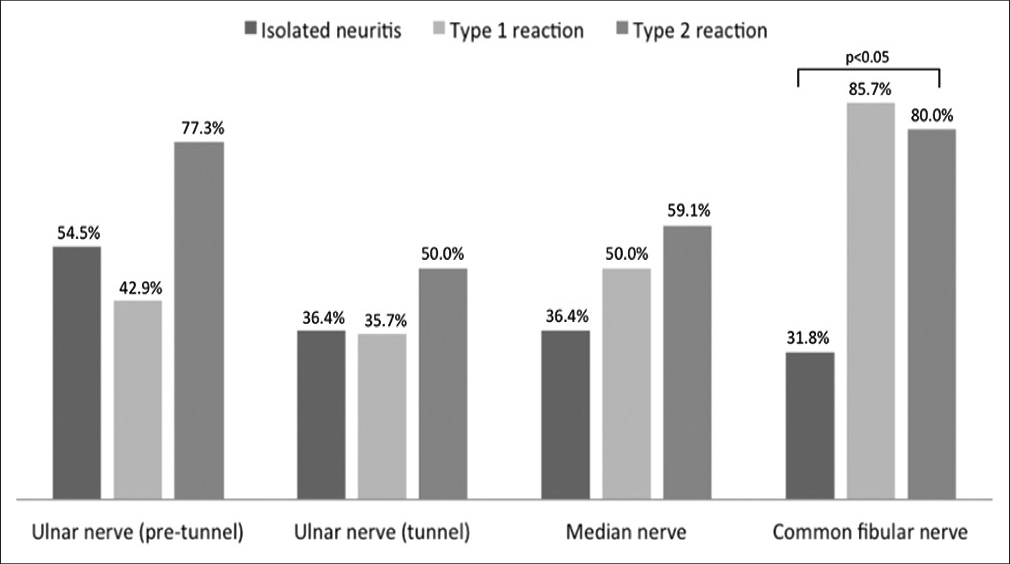
- Frequency of poor cross-sectional area outcomes for patients with isolated neuritis, type 1 and type 2 reactions
Sensory testing and ultrasonography results were not significantly different between patients with positive or negative slit-skin smears and between patients with or without diabetes. There was no correlation between poor sensory and poor cross-sectional area outcomes for the studied nerves.
This is the first study investigating ultrasonography findings and their correlation with sensory testing in leprosy neuritis treated with pulse therapy. Despite the severity of nerve involvement of our patients, we found that pulse was effective in preserving nerve function. In addition, it allowed prednisone dose reduction/withdrawal even in patients with chronic steroid use before pulses. As expected, ultrasonography findings showed that pulse therapy was not effective in improving nerve enlargement, which may persist despite antibacterial and anti-reaction treatments.3 However, it reduced hypervascularity/ inflammation as detected by Doppler signal.
The only randomised controlled double blind trial comparing pulse therapy and oral steroids showed better sensory testing results in the pulse group at day 29 after infusion but no beneficial effect by day 337.5 In that trial, patients received a single pulse, without subsequent monthly infusions, likely hindering the potential benefit. Further, approximately 20% of the patients in that sample did not have nerve function impairment,5 while we only included patients with severe and/or recalcitrant neuritis.
The sample size and retrospective design are drawbacks of our study. Although diabetic neuropathy could be a confounding factor, results did not differ significantly between the patients with and without diabetes. Pulse therapy may be an interesting option for neuritis treatment in diabetic patients, allowing for smaller daily steroid doses, thus contributing to better metabolic control.
In conclusion, pulse therapy was effective in preserving sensibility and allowing reduction of oral steroid doses. Nerve ultrasonography can be an adjuvant tool for monitoring the inflammatory process in leprosy neuritis.
Declaration of patient consent
Institutional Review Board (IRB) permission obtained for the study.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- New sonographic measures of peripheral nerves: A tool for the diagnosis of peripheral nerve involvement in leprosy. Mem Inst Oswaldo Cruz. 2013;108:257-62.
- [CrossRef] [PubMed] [Google Scholar]
- Asymmetric nerve enlargement: A characteristic of leprosy neuropathy demonstrated by ultrasonography. PLoS Negl Trop Dis. 2015;9:e0004276.
- [CrossRef] [PubMed] [Google Scholar]
- Ultrasonography of leprosy neuropathy: A longitudinal prospective study. PLoS Negl Trop Dis. 2016;10:e0005111.
- [CrossRef] [PubMed] [Google Scholar]
- Diretrizes Para Vigilância, Atenção e Eliminação da Hanseníase Como Problema de Saúde Pública: Manual Técnico-operacional. 2016. Ministério da Saúde, Brasília, DF, Brazil. Available from: http://www.portalarquivos2.saude.gov.br/images/pdf/2016/fevereiro/04/diretrizes-eliminacao-hanseniase-4fev16-web.pdf [Last accessed on 2021 Jun 20]
- [Google Scholar]
- A phase two randomised controlled double blind trial of high dose intravenous methylprednisolone and oral prednisolone versus intravenous normal saline and oral prednisolone in individuals with leprosy Type 1 reactions and/or nerve function impairment. PLoS Negl Trop Dis. 2011;5:e1041.
- [CrossRef] [PubMed] [Google Scholar]





