Translate this page into:
Multiple isolated cutaneous plexiform schwannomas
Correspondence Address:
Enas A. S. Attia
Department of Dermatology, Venereology and Andrology, Faculty of Medicine, Ain Shams University, Cairo, PO: 11566
Egypt
| How to cite this article: Attia EA, Yassin M, Lasheen MA, Salem SA, Khafagy NH. Multiple isolated cutaneous plexiform schwannomas. Indian J Dermatol Venereol Leprol 2011;77:594-596 |
Abstract
Plexiform schwannoma is a rare neurogenic tumor, arising from skin and subcutaneous tissue. The presence of multiple schwannomas suggests a possible association with neurofibromatosis type 2 (NF2). A 50-year old male patient presented with multiple papulo-nodular cutaneous lesions on both arms and forearms. Histopathological examination revealed a dermal multinodular pattern of well-circumscribed masses of closely packed cells, with peripheral myxoid tissue, well-encapsulated in a thin collagenous capsule. S-100 immunohistochemical staining was diffusely and strongly positive. Neuron-specific enolase was positive, confirming a neural tissue tumor. An audiogram and Magnetic Resonance Imaging (MRI) of cerebro-pontine angle showed no detected abnormality, excluding acoustic neuroma. Thus, we present a case of multiple bilateral isolated cutaneous plexiform schwannomas, not associated with NF2. Multiple plexiform schwannomas is a very rare entity, distinct from neurofibromatosis (NF), and being confined to the dermis is even more rarely reported.Introduction
A neural tumor is defined as any tumor that arises from neural tissue or its coverings. Schwannomas and neurofibromas are the two most common types. Schwannomas are very homogenous tumors and consist of Schwann cells only. They develop on the outside of the nerve, but may push it aside or against adjacent structures, causing damage. Neurofibromas are very heterogenous tumors arising by a combined proliferation of several elements of a peripheral nerve, including axons, schwann cells, fibroblasts, perineural cells and endoneurium. They infiltrate the nerve and displace the individual nerve fibers. [1] Both tumors can develop plexiform or multinodular subtypes. Plexiform schwannoma is a rare neurogenic tumor, often arising from skin and subcutaneous tissue. [2] Differentiation from plexiform neurofibroma is important, because the latter is virtually pathognomonic of neurofibromatosis type 1 (NF1) and has a propensity for malignant transformation. The presence of multiple schwannomas in a single patient suggests tumorogenesis and a possible association with one of several syndromes, most commonly NF2. [3] Here we present a case with multiple bilateral isolated cutaneous plexiform schwannomas not associated with NF2.
Case Report
A 50-year old male patient presented with multiple asymptomatic, skin-colored papulo-nodular cutaneous lesions on both arms and forearms of seven months duration [Figure - 1]. There were no abnormal neurological or ophthalmological signs on examination. The cutaneous lesions were not typical in appearance for distribution for NF1, and there were no café au lait patches, axillary freckling, or obvious Lisch nodules. Two excisional skin biopsies were taken from those lesions and histopathological examination revealed an upper dermal multinodular pattern of well-circumscribed masses formed of closely-packed cells, well-encapsulated in a thin collagenous capsule [Figure - 2]a. Higher magnification showed a peripheral myxoid tissue surrounding masses of spindle-shaped cells having elongated wavy nuclei [Figure - 2]b. Immunohistochemical staining for actin was negative, excluding the possibility of smooth muscle tumor. S-100 immunohistochemical staining was diffusely and strongly positive, suggesting a neural tissue tumor [Figure - 2]c. Staining for Neuron-specific enolase (NSE) was done and it was positive [Figure - 2]d, which together with the above mentioned histopathological findings supported the diagnosis of a neural tissue tumor.
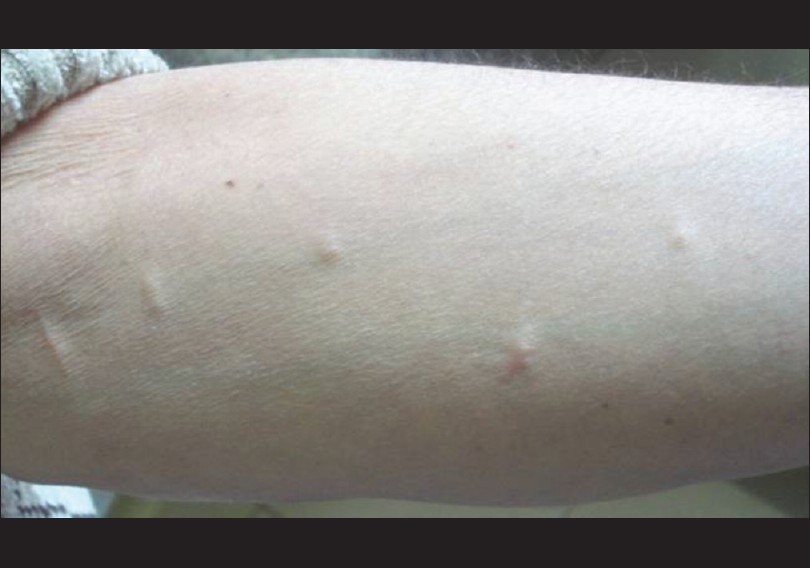 |
| Figure 1: Multiple small skin-coloured papulo-nodular cutaneous lesions on the forearm of the patient in the case study |
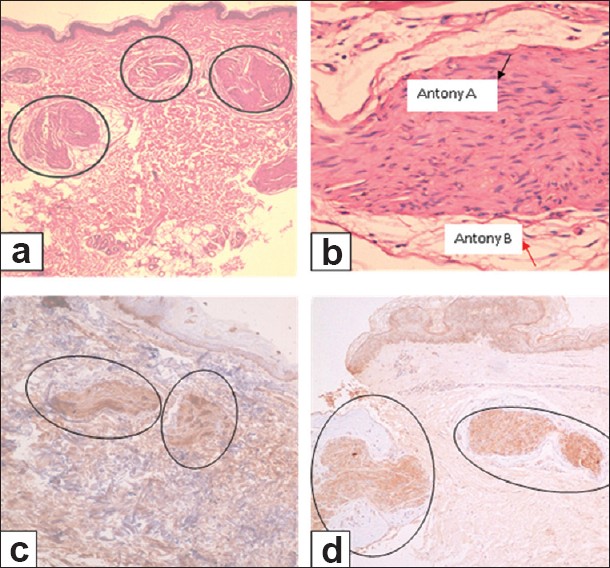 |
| Figure 2: Skin excisional biopsy from forearm lesion. (a) Multinodular pattern in the upper dermis of well circumscribed masses of closely-packed cells, well-encapsulated in a thin collagenous capsule (H and E, ×40). (b) Higher magnification showing spindle-shaped cells containing elongated wavy nuclei with peripheral myxoid tissue. Black arrow denotes Antoni A areas and red arrow denotes Antoni B areas (H and E, ×400). (c) Tumor masses showing diffusely and strongly positive S-100 (immunhistochemical staining, ×100). (d) Tumor masses showing positive staining for neuron-specific enolase (immunhistochemical staining, ×100) |
Based on absent Tinel′s sign (pain upon percussion of the lesion) in a neural tumor originating from superficial nerves, and the histopathological findings of a multinodular pattern composed of spindle cells in a loose myxoid stroma, with characteristic zonation of peripheral myxoid elements and central hypercellular elements, i.e. target sign, [4] the diagnosis of multiple plexiform schwannomas was established. In contrast, neurofibroma would display absent capsule, lack of Antoni A and B areas of schwannoma, and the presence of weak, patchy S-100 positivity. [4]
As plexiform schwannoma could be associated with NF2, [3] an audiogram and Magnetic Resonance Imaging (MRI) of the cerebello-pontine angle were done, but no abnormality could be detected, excluding acoustic neuroma, and hence NF2.
Clinical-imaging examination (brain and spinal MRI) ruled out the possibility of meningiomas, gliomas or schwannomas elsewhere.
Discussion
Plexiform schwannoma is composed exclusively of Schwann cells exhibiting a plexiform or multinodular arrangement. Masson was the first to recognize this variant, which he called "plexiform schwannogliosis" [5] Approximately 5% of schwannomas develop into plexiform schwannomas. These tumors may occur as solitary or multiple lesions. Moreover, they may be localized to a single anatomic site or diffusely distributed. Typical lesions are observed as slowly growing, multinodular, well-circumscribed, dermal, or subcutaneous tumors of the trunk, extremities, or head and neck. Some tumors are painful or tender. [4] The majority of classic schwannomas are solitary lesions, which often arise in the major peripheral nerves. Classic schwannomas can exhibit a positive Tinel′s sign, if originated in major peripheral nerves. However, of the approximately 100 cases documented in the literature, very few examples exist in which plexiform schwannomas originated in major peripheral nerves. [6] Therefore, as with the present case, plexiform schwannomas rarely produce a positive Tinel′s sign.
Pathologically, plexiform schwannoma consists of Antoni A and Antoni B elements, as in classic schwannoma. The target sign corresponds to the zonation of peripheral-myxoid Antoni B elements and central-hypercellular Antoni A elements. [4] Some plexiform schwannomas have cellular appearance; that mimics malignant peripheral nerve sheath tumor, and can arise as a deep-seated mass. [7] Schwannomatosis was first reported in 1973 as neurofibromatosis type 3 (NF3). [8] Then, the term "schwannomatosis" was used to describe a phenotype of multiple cutaneous schwannomas in absence of bilateral vestibular schwannomas. [9] Later on, Jacoby et al.[10] proposed clinical criteria for the diagnosis of schwannomatosis. They suggested two or more pathologically proven schwannomas and lack of radiographic findings of vestibular tumors at an age more than 18 years, as an evidence of definite schwannomatosis. However, Mac Collin et al. (2005) [11] settled criteria for definite schwannomatosis if the patient has two or more non-intradermal schwannomas, is older than age 30 years, lacks evidence of vestibular schwannoma on high quality MRI scan, and does not have a known constitutional NF2 mutation. Nevertheless, schwannomatosis can demonstrate somatic NF2 mutations within tumors and in about 10% of sporadic cases, germline mutations of SMARCB1/INI1. [12] If MRI of the brain is not available, then a presumptive diagnosis may be made if the patient has two or more pathologically proven schwannomas and no clinical symptoms at age more than 30 years. [11] It seems that multiple non cutaneous schwannomas, without acoustic tumors or other signs of NF1 or NF2, is characteristic. Thus, being restricted to intradermal lesions, our patient may not fulfill the diagnosis of schwannomatosis.
Multiple schwannomas in the same individual may suggest NF2. [3] Two-thirds of NF2-affected individuals will develop schwannomas and they may precede vestibular tumors. Various authors reported individuals with multiple schwannomas who do not show evidence of vestibular neuroma or other features of NF2. They suggest that it is a distinct entity from other forms of NF. [11] It is important to consider genetic testing, as molecular diagnosis rules out NF2. A very small fraction of patients with spinal or peripheral schwannomas are managed neurosurgically. These patients commonly undergo multiple operative procedures. [6] Our patient refused more surgical intervention.
In conclusion, Schwannoma is a neural tumor distinct from neurofibroma. The plexiform type of schwannoma is rare, and the multiple plexiform schwannomas is even very rare. Lesions confined to the dermis are the rarest. Such cases are distinct from NF provided that the presence of acoustic neuroma is excluded. It is important to consider genetic testing, as molecular diagnosis definitely rules out NF2.
| 1. |
Rameh C, Husseini S, Tawil A, Fuleihan N, Hadi U. Solitary plexiform neurofibroma of the nasal tip: Case report and review of the literature. Int J Pediatr Otorhinolaryngol 2007;2:116-9.
[Google Scholar]
|
| 2. |
Haraida S, Nerlich AG, Bise K, Wiest I, Schleicher E. Comparison of various basement membrane components in benign and malignant peripheral nerve tumors. Virchows Arch A Pathol Anat Histopathol 1992;421:331-8.
[Google Scholar]
|
| 3. |
Val-Bernal JF, Figols J, Vázquez-Barquero A . Cutaneous plexiform schwannoma associated with neurofibromatosis type 2. Cancer 1995;76:1181-6.
[Google Scholar]
|
| 4. |
Berg JC, Scheithauer BW, Spinner RJ, Allen CM, Koutlas IG. Plexiform schwannoma: A clinicopathologic overview with emphasis on the head and neck region. Hum Pathol 2008;39:633-40.
[Google Scholar]
|
| 5. |
Masson P. Human tumors: Histology, diagnosis, and technique. Detroit: Wayne State University Press; 1970.
[Google Scholar]
|
| 6. |
Hébert-Blouin MN, Amrami KK, Scheithauer BW, Spinner RJ. Multinodular/plexiform (multifascicular) schwannomas of major peripheral nerves: An underrecognized part of the spectrum of schwannomas. J Neurosurg 2010;112:372-82.
[Google Scholar]
|
| 7. |
Agaram NP, Prakash S, Antonescu CR. Deep-seated plexiform schwannoma: A pathologic study of 16 cases and comparative analysis with the superficial variety. Am J Surg Pathol 2005;29:1042-8.
[Google Scholar]
|
| 8. |
Nimura M. Neurofibromatosis. Rinsho Derma 1973;15:653-63.
[Google Scholar]
|
| 9. |
Shishiba T, Niimura M, Ohtsuka F, Tsuru N. Multiple cutaneous neurilemmomas as a skin manifestation of neurilemmomatosis. J Am Acad Dermatol 1984;10:744-54.
[Google Scholar]
|
| 10. |
Jacoby LB, Jones D, Davis K, Kronn D, Short MP, Gusella J, et al. Molecular analysis of NF2 tumor suppressor gene in Schwannomatosis. Am J Hum Genet 1997;61:1293-302.
[Google Scholar]
|
| 11. |
MacCollin M, Chiocca EA, Evans DG, Friedman JM, Horvitz R, Jaramillo D, et al. Diagnostic criteria for schwannomatosis. Neurology 2005;64:1838-45.
[Google Scholar]
|
| 12. |
Rousseau G, Noguchi T, Bourdon V, Sobol H, Olschwang S. SMARCB1/INI1 germline mutations contribute to 10% of sporadic schwannomatosis. BMC Neurology 2011;11:9.
[Google Scholar]
|
Fulltext Views
4,914
PDF downloads
1,842





