Translate this page into:
Optimal biologic dosing in management of obese patients with psoriasis
Corresponding author: Dr. Shekhar Neema, Department of Dermatology, Armed Forces Medical College, Pune - 411 040, Maharashtra, India. shekharadvait@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Neema S, Pathania V, Pudasaini N, Subramaniyan R. Optimal biologic dosing in management of obese patients with psoriasis. Indian J Dermatol Venereol Leprol 2021;87:424-6.
Sir,
Psoriasis is a common chronic inflammatory skin disorder affecting 0.4%–2% of the Indian population.1 It is associated with various comorbidities like arthritis, metabolic syndrome, obesity, diabetes mellitus, coronary artery disease and depression. Obesity is more commonly seen in patients with severe psoriasis and is more common in younger patients (<35 years) as compared to older patients (>65 years).2 In obese patients, systemic drugs like methotrexate and acitretin have a higher risk of hepatotoxicity and cyclosporine results in higher trough levels leading to a higher risk of nephrotoxicity. Dosing in biologics like etanercept, adalimumab and secukinumab is independent of weight and often leads to poor response in obese patients.
A 65-year-old male, having chronic plaque psoriasis for the last 23 years, presented to department of Dermatology, Command Hospital (Southern Command), Pune, with acute exacerbation of 8 weeks. In the last 23 years, the patient had multiple episodes of erythroderma and had been treated with methotrexate (15–25 mg per week, cumulative total dose-6800 mg), cyclosporine, acitretin and phototherapy with partial relief. He was also prescribed injection etanercept 50 mg subcutaneously, twice a week for 12 weeks, followed by once a week for 1 year and injection secukinumab 300 mg on day 0, every week for 4 weeks and monthly for 18 months. The patient had partial relief to prescribed drugs but did not achieve complete remission in the last 6 years. Clinical examination revealed a weight of 100 kg with body mass index of 32.18 kg/m2. Dermatological examination revealed 70% body surface area involvement, with well defined, erythematous, scaly plaques with psoriasis assessment severity index (PASI) score of 28.4 [Figure 1].The rest of the examination was within normal limits. Baseline workup including Mantoux test, interferon-gamma release assay, radiograph of the chest and viral markers were negative. We administered injection infliximab 500 mg (5 mg/kg) on day 0, week 2, 6 and every 8 weeks thereafter along with methotrexate 7.5 mg/week. He achieved a complete remission at 6 weeks and is maintaining remission at 6 monthly follow-up [Figure 2]. We plan to continue 8 weekly infliximab infusion.
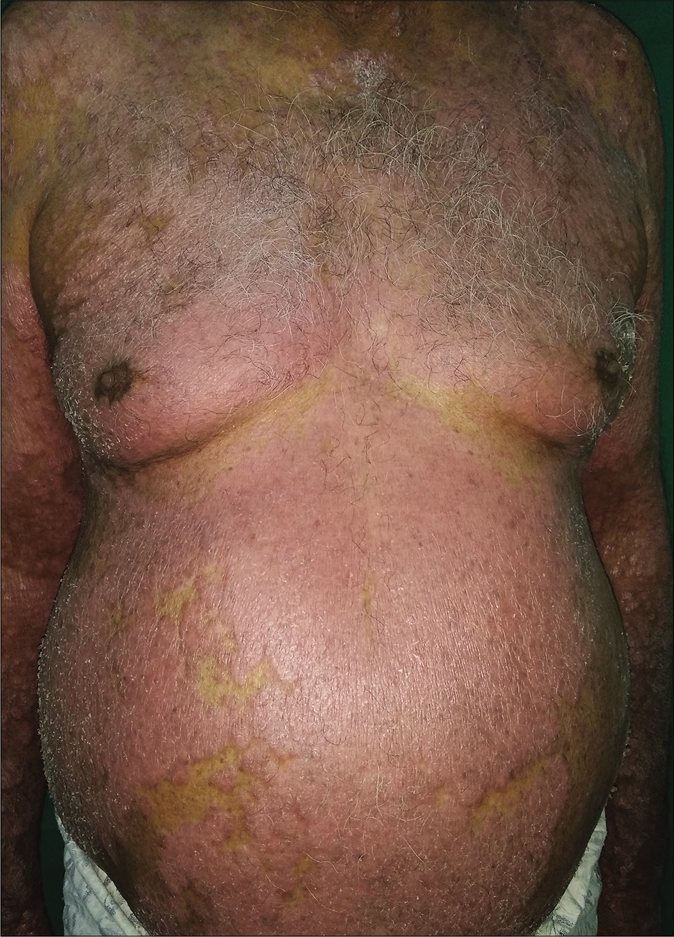
- Involvement of trunk with erythematous, scaly plaques
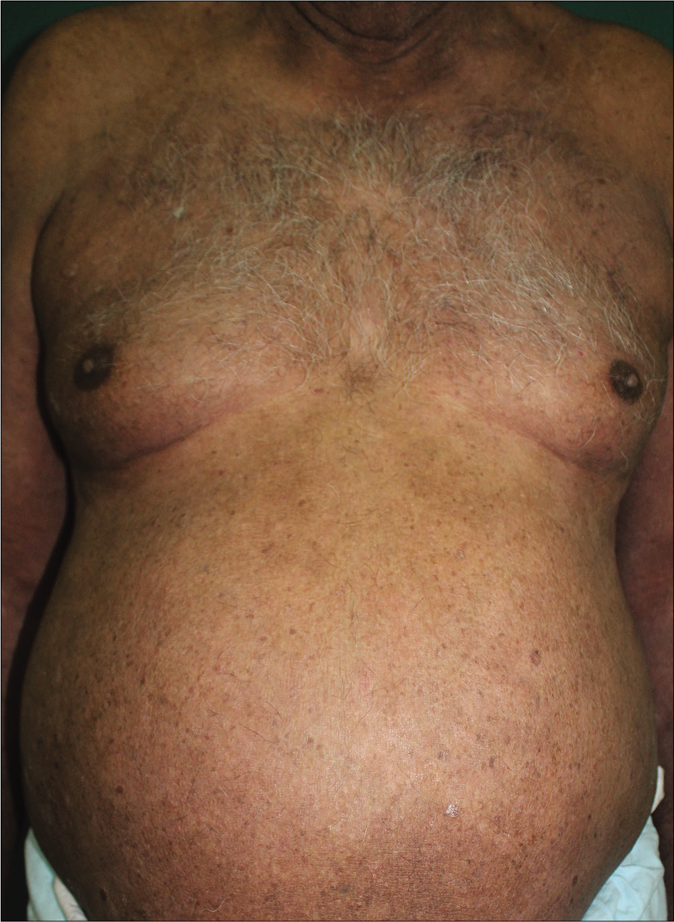
- Complete clearance 6 weeks after starting infliximab
The available biologics have differential efficacy with secukinumab and infliximab being the most efficacious (PASI 75: 81% and 80% respectively), followed by adalimumab (PASI 75: 71%) and etanercept (PASI 75: 49%). Infliximab has weight-based dosing while adalimumab, etanercept and secukinumab are administered on fixed-dose schedule irrespective of weight. In patients on etanercept, 41% and 25% of patients achieved PASI 75 in patients weighing less than 89 kg and more than 89 kg, respectively. PASI 75 was achieved in 74% patients weighing 40–78 kg as compared to 62% in those more than 105 kg in patients on adalimumab.3 The lower response rate was seen with higher weight in patients on secukinumab.4 These low responses may be due to higher volume of distribution and faster drug clearance in obese patients. Efficacy of infliximab is consistent across patients with various body mass indexes due to weight-based dosing.5 Development of antidrug antibodies, while using infliximab is reduced by the concomitant use of low-dose methotrexate (7.5 mg/week). The excellent response seen in our patient, who was unresponsive to other treatments, is possibly due to weight-based dosing of infliximab. However, it may also be due to the higher efficacy of infliximab per se or the combination of infliximab with methotrexate.
In conclusion, the management of obese patients with psoriasis can be challenging. Conventional systemic therapy and biologics with fixed dosing may result in a suboptimal response. Infliximab is an efficacious biologic and weight-based dosing may help achieve clearance even in obese patients.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- Psoriasis in India: Prevalence and pattern. Indian J Dermatol Venereol Leprol. 2010;76:595-601.
- [CrossRef] [PubMed] [Google Scholar]
- Prevalence of cardiovascular risk factors in patients with psoriasis. J Am Acad Dermatol. 2006;55:829-35.
- [CrossRef] [PubMed] [Google Scholar]
- Obesity and psoriasis: From the Medical Board of the National Psoriasis Foundation. J Am Acad Dermatol. 2010;63:1058-69.
- [CrossRef] [PubMed] [Google Scholar]
- Characterization of responder groups to secukinumab treatment in moderate to severe plaque psoriasis. J Dermatolog Treat. 2020;31:769-75.
- [CrossRef] [PubMed] [Google Scholar]
- Consistency of infliximab response across subgroups of patients with psoriasis: Integrated results from randomized clinical trials. J Am Acad Dermatol. 2006;54(Suppl 1):AB215.
- [CrossRef] [Google Scholar]





