Translate this page into:
Psoriasiform reactions during treatment with abatacept
Correspondence Address:
Elena Conde-Montero
Department of Dermatology, Hospital Universitario Gregorio Mara��n, Madrid
Spain
| How to cite this article: Conde-Montero E, Baniandr�s-Rodr�guez O, Mendoza-Cembranos MD, Horcajada-Reales C, Su�rez-Fern�ndez R. Psoriasiform reactions during treatment with abatacept. Indian J Dermatol Venereol Leprol 2014;80:92-93 |
Sir,
Abatacept is a biological immune modifier agent that is currently used for the treatment of rheumatoid arthritis (RA). Cutaneous adverse effects caused by this drug are uncommon. We report two cases of psoriasiform reactions during treatment with abatacept.
Case 1: A 56-year-old man with RA in treatment with abatacept for 1 year with little response, presented with a 4-month a history of psoriasiform lesions affecting palms, soles [Figure - 1]a and his scalp [Figure - 1]b. He had no personal or family history of psoriasis. Abatacept was not discontinued; however, he achieved complete remission with topical steroids and calcipotriol.
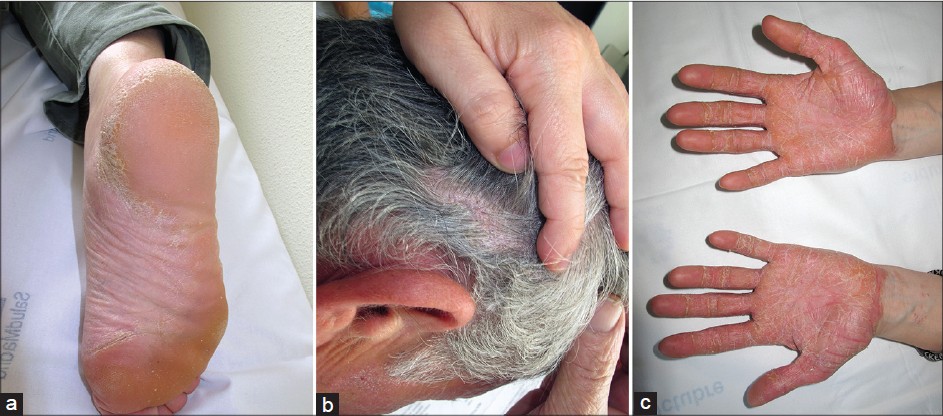 |
| Figure 1: (a) Case 1: Plantar hyperkeratotic plaques. (b) Case 1: Scaly plaques on the scalp. (c) Case 2: Palmar erythematous hyperkeratotic plaques |
Case 2: A 25-year-old woman was followed for a 10-year-history of moderate psoriasis, in sustained remission for years and recalcitrant psoriatic arthritis. Leflunomide, cyclosporine, methotrexate, infliximab and adalimumab had all been unsatisfactory for controlling her arthritis. Two months after abatacept therapy was initiated, she reported a flare of psoriasis, with erythematous hyperkeratotic plaques on palms [Figure - 1]c and soles and scaly lesions on her scalp. Topical steroids and calcipotriol were initiated without response. After discontinuing abatacept and initiating ustekinumab, the cutaneous lesions improved.
Triggering or worsening of psoriasis has been traditionally reported in patients treated with each of the three currently available tumor necrosis factor (TNF)-α blocking agents (infliximab, etanercept and adalimumab). [1] In most cases, psoriasis appearing during the anti-TNF-α therapy shows a pustular palmoplantar pattern. Considering the evidence-based therapeutic effect of these agents in psoriasis, induction of these lesions represents a paradoxical reaction, whose underlying mechanism remains elusive. [2] It has been suggested that the increased production of interferon-g after TNF-α blockage might play a role. [3]
Abatacept is a chimeric protein, which binds to CD80 and CD86 on antigen-presenting cells and inhibits the engagement of CD28 on T-cells thus preventing effective T-cell activation. This cytotoxic T-lymphocyte-associated antigen 4 (CTLA-4)-immunoglobulin G1 is currently approved for the treatment of RA. [4] Few cases of paradoxical psoriasis during treatment with abatacept have been reported. [2],[4],[5] In a meta-analysis of 13 relevant trials, four of 1332 RA patients who received abatacept as monotherapy as well as 13 of 1945 patients treated with abatacept plus disease-modifying antirheumatic drugs developed psoriasis as an adverse event. [4]
Our first case represents an onset of psoriatic lesions in the absence of family history or other triggering factors, 8 months after initiating abatacept. The second patient developed an acute worsening of her psoriasis within the first two months of treatment with abatacept and achieved prompt recovery after drug withdrawal.
The underlying mechanism remains unclear. The new targeted biological therapies provide us with the possibility of blocking a specific biological activity underlying the primary pathological condition. However, the target may be involved in multiple complex immunological pathways, resulting in an unexpected and in some cases paradoxical, response. [2] It has been suggested that the interference with CTLA-4 signals in regulatory T-cells might result in the impaired suppressive functions of those cells and in the exacerbation of Th17 immunity. [5] Further research is needed so as to achieve a better understanding of this phenomenon.
| 1. |
de Gannes GC, Ghoreishi M, Pope J, Russell A, Bell D, Adams S, et al. Psoriasis and pustular dermatitis triggered by TNF-{alpha} inhibitors in patients with rheumatologic conditions. Arch Dermatol 2007;143:223-31.
[Google Scholar]
|
| 2. |
Brunasso AM, Laimer M, Massone C. Paradoxical reactions to targeted biological treatments: A way to treat and trigger? Acta Derm Venereol 2010;90:183-5.
[Google Scholar]
|
| 3. |
Seneschal J, Milpied B, Vergier B, Lepreux S, Schaeverbeke T, Taïeb A. Cytokine imbalance with increased production of interferon-alpha in psoriasiform eruptions associated with antitumour necrosis factor-alpha treatments. Br J Dermatol 2009;161:1081-8.
[Google Scholar]
|
| 4. |
Konsta M, Rallis E, Karameris A, Stratigos A, Sfikakis PP, Iliopoulos A. Psoriasiform lesions appearing in three patients with rheumatoid arthritis during therapeutic administration of abatacept, a selective inhibitor of T-cell costimulation. J Eur Acad Dermatol Venereol 2012;26:257-8.
[Google Scholar]
|
| 5. |
Kato K, Satoh T, Nishizawa A, Yokozeki H. Psoriasiform drug eruption due to abatacept. Acta Derm Venereol 2011;91:362-3.
[Google Scholar]
|
Fulltext Views
3,453
PDF downloads
3,006





