Translate this page into:
Psoriasis increases the risk of concurrent inflammatory bowel disease: A population-based nationwide study in Korea
2 Institute of Health and Environment, Seoul National University, Seoul, Korea
3 Department of Dermatology, College of Medicine, The Catholic University of Korea, Seoul, Korea
4 Department of Dermatology, Seoul National University Hospital, Seoul, Korea
5 Department of Internal Medicine, SMG-SNU Boramae Medical Center, Seoul, Korea
6 Department of Dermatology, SMG-SNU Boramae Medical Center, Seoul, Korea
Correspondence Address:
Hyun-Sun Park
20 Boramae-Ro-5-Gil, Dongjak-Gu, Seoul 07061
Korea
| How to cite this article: Lee JY, Kang S, Bae JM, Jo SJ, Koh SJ, Park HS. Psoriasis increases the risk of concurrent inflammatory bowel disease: A population-based nationwide study in Korea. Indian J Dermatol Venereol Leprol 2019;85:145-152 |
Abstract
Background: The epidemiology of the association between psoriasis and inflammatory bowel disease is poorly defined and remains controversial.
Aim: To evaluate the prevalence of inflammatory bowel disease in patients with psoriasis compared with the general population.
Methods: We searched the nationwide health claims database between 2011 and 2015 and evaluated the prevalence of inflammatory bowel disease, including Crohn's disease and ulcerative colitis.
Results: Prevalence of inflammatory bowel disease, Crohn's disease and ulcerative colitis in patients with psoriasis vs the general population in 2011 were 0.16, 0.05 and 0.12% vs 0.08, 0.03 and 0.06%, respectively, which increased significantly with time between 2011 and 2015. Patients with psoriasis consistently revealed higher standardized prevalence (age and sex adjusted) of inflammatory bowel disease, Crohn's disease and ulcerative colitis compared with the general population. Subgroup analysis revealed the highest risk of prevalent inflammatory bowel disease in patients younger than 19 years (crude odds ratio 5.33, 95% confidence interval 3.74–7.59). Severe psoriasis demonstrated higher odds of inflammatory bowel disease (odds ratio 2.96, 95% confidence interval 2.54–3.45) than mild psoriasis (odds ratio 1.68, 95% confidence interval 1.51–1.88).
Limitations: Limited data for doing adjustment and cross-sectional study design.
Conclusions: Psoriasis patients revealed higher risk of inflammatory bowel disease. In particular, young patients and those with severe psoriasis may require closer monitoring and comprehensive management.
Introduction
The association between psoriasis and inflammatory bowel disease has been repeatedly reported, which can be explained by common genetic and inflammatory pathways including tumor necrosis factor-alpha or interleukin-17.[1],[2],[3],[4] However, the epidemiology of this relationship is poorly defined and remains controversial.[5] Research from the United States, Germany and Israel reported increased prevalence of Crohn's disease or ulcerative colitis among patients with psoriasis compared with healthy subjects.[6],[7],[8],[9],[10] On the other hand, research in Taiwan reported that psoriasis was not associated with increased risk of Crohn's disease.[11] Studies on newly incident inflammatory bowel disease associated with psoriasis are also conflicting. A nationwide Danish cohort study[12] revealed increased risk of both incident Crohn's disease and ulcerative colitis associated with psoriasis, whereas a US study[13] in women nurses reported increased risk of Crohn's disease, but not ulcerative colitis. Some of these previous studies had limitations, however, including limited populations[6],[8],[9],[13] or specific patient groups (e.g. women nurses),[13] which do not provide robust information of real-world patients. Given the concerns regarding inflammatory bowel disease in patients with psoriasis as a systemic inflammatory disease, we searched the nationwide health claims database of Korea and aimed to identify the risk of inflammatory bowel disease in patients with psoriasis compared with the general population.[14]
Methods
This study was exempt from approval by the Institutional Review Board.
Data source
We used the database of the Health Insurance Review and Assessment Service, an independent Government agency responsible for conducting reviews of health claims data and evaluating the appropriateness of medical benefits submitted by healthcare organizations. Health Insurance Review and Assessment Service's claims data include all medical utilization covered by insurance program of entire Korean population. National Health Insurance program covers the majority of the population and Medical Aid manages the remaining 3% (special population with low socioeconomic status).
Study population and operational definitions of psoriasis and inflammatory bowel disease
Patients with inflammatory bowel disease refer to those who have International Classification of Diseases, 10th revision, diagnostic codes of ulcerative colitis (K51.0, K51.1, K51.3, K51.5, K51.8, K51.9) or Crohn's disease (K50.x) from 2011 through 2015. Patients with psoriasis refer to those who have a diagnostic code of L40.x during the same period. We recategorized patients with psoriasis in 2015 into the severe group if they received phototherapy, systemic agents (acitretin, cyclosporine, methotrexate) or biologic agents (infliximab, etanercept, adalimumab, ustekinumab). Otherwise, they were classified into the mild group.
Study outcomes
The primary endpoint was annual prevalence of inflammatory bowel disease in patients with psoriasis and the general population, respectively. The secondary outcome was risk of inflammatory bowel disease in psoriasis subgroups according to age, sex, insurance type and psoriasis severity.
Statistical analysis
Annual prevalence was calculated in two ways: (1) crude prevalence without adjustment and (2) standardized number per 100,000 population after adjusting for sex and age based on the 2013 population data from the Korea National Statistics Office.
Frequency analyses were conducted to describe the distribution of prevalence. We calculated crude odds ratios to identify the relative risk of prevalent inflammatory bowel disease in patients with psoriasis vs the general population. Next, we performed time-trend analyses using the Chi-squared test for trend method to investigate whether prevalence of inflammatory bowel disease increased over time. Lastly, the Cochran–Armitage test was used to identify whether different time trends existed between patients with psoriasis and the general population. All statistical tests were performed using SAS version 9.4 Enterprise Guide (SAS Institute, Inc., Cary, NC, USA). All tests were two-sided and a P value of < 0.05 was considered statistically significant.
Results
Characteristics of patients with psoriasis and the general population
The baseline characteristics of the study population are summarized in [Table - 1]. We identified a total of 50,111,476 patients, including 219,429 patients with psoriasis, in 2011. There were 43,689 patients with inflammatory bowel disease in the general population and 357 patients with inflammatory bowel disease among patients with psoriasis in 2011. These numbers increased between 2011 and 2015. Approximately 60% of subjects were male.
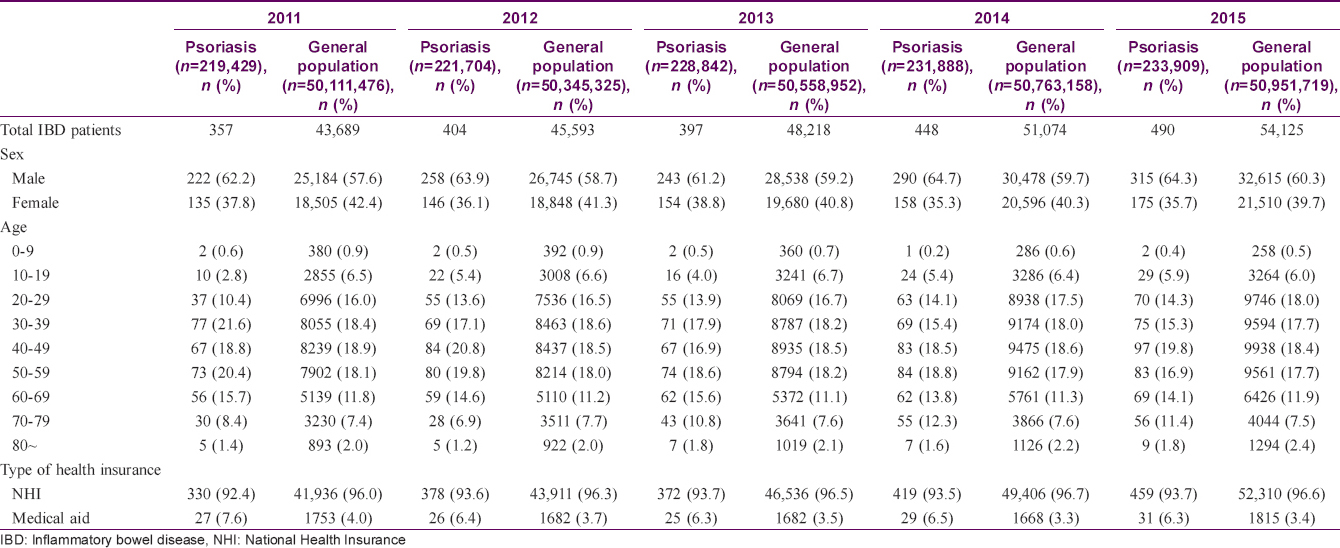
Prevalence of inflammatory bowel disease in patients with psoriasis and the general population
Prevalence of inflammatory bowel disease in patients with psoriasis and the general population were 0.16–0.21% and 0.08–0.11%, respectively [Table - 2]. Prevalence in each group increased significantly between 2011 and 2015 (P = 0.002 in patients with psoriasis; P < 0.0001 in the general population). There was no significant difference in increasing trend between groups (P = 0.2049). When inflammatory bowel disease was classified into Crohn's disease and ulcerative colitis, similar patterns were revealed.
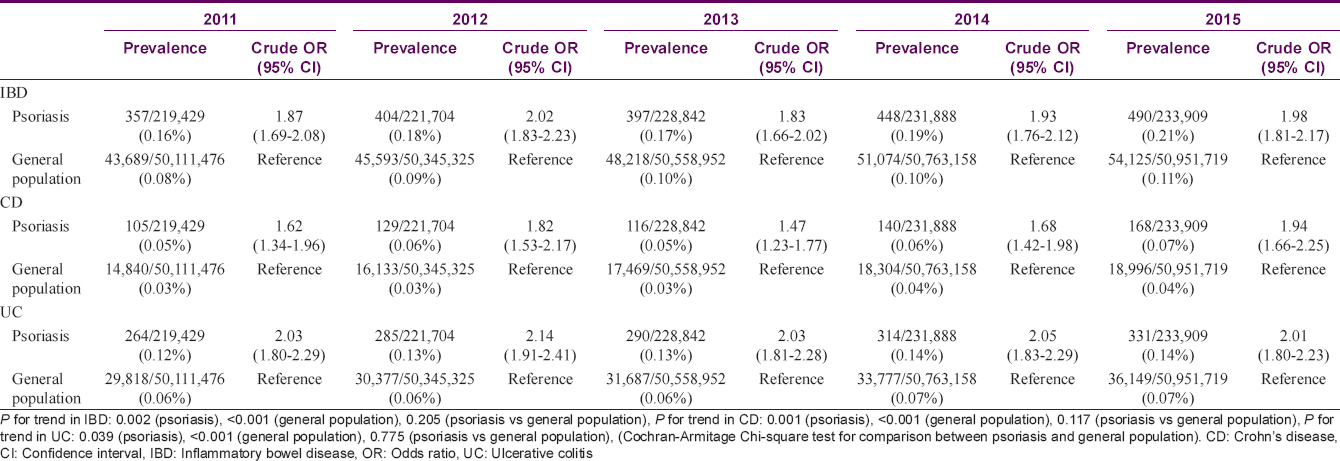
Patients with psoriasis demonstrated significantly increased likelihood of prevalent inflammatory bowel disease during the study period (crude odds ratio, 1.87–2.02). Again, when inflammatory bowel disease was classified into Crohn's disease and ulcerative colitis, both entities revealed increased likelihood [Table - 2].
Next, we calculated age- and sex-adjusted standardized prevalence of inflammatory bowel disease, Crohn's disease and ulcerative colitis per 100,000 person during the study period. Patients with psoriasis consistently revealed higher standardized prevalence of inflammatory bowel disease, Crohn's disease and ulcerative colitis compared with the general population [Figure - 1].
 |
| Figure 1: |
Association between inflammatory bowel disease and psoriasis in psoriasis subgroups
Subgroup analyses [Table - 3] were performed using the data from 2015. Both sexes demonstrated increased risk of inflammatory bowel disease, Crohn's disease and ulcerative colitis. Although psoriasis increased the risk of inflammatory bowel disease in all age groups, patients younger than 19 years of age revealed the highest likelihood of inflammatory bowel disease (odds ratio, 5.33; 95% confidence interval, 3.74–7.59). Furthermore, Crohn's disease (odds ratio, 5.96; 95% confidence interval, 4.02–8.85) revealed a stronger association than ulcerative colitis (odds ratio, 3.78; 95% confidence interval, 1.80–7.95) in this age group. Severe psoriasis demonstrated higher likelihood of inflammatory bowel disease (odds ratio, 2.96; 95% confidence interval, 2.54–3.45) compared with mild psoriasis (odds ratio, 1.68; 95% confidence interval, 1.51–1.88). As adalimumab and infliximab are commonly used to treat both inflammatory bowel disease and psoriasis in Korea, we also performed an analysis after excluding patients who were treated with these medications. Severe psoriasis still demonstrated increased risk of ulcerative colitis (odds ratio, 2.08; 95% confidence interval, 1.66–2.59) but not Crohn's disease (odds ratio, 0.97; 95% confidence interval, 0.62–1.52). Similarly, patients with psoriatic arthritis revealed increased risk of inflammatory bowel disease (odds ratio, 2.67; 95% confidence interval, 1.70–4.19), especially ulcerative colitis (odds ratio, 3.20; 95% confidence interval, 2.04–5.01). Patients with psoriasis demonstrated increased prevalence of inflammatory bowel disease, Crohn's disease and ulcerative colitis, irrespective of socioeconomic status (National Health Insurance or Medicaid).

Discussion
Psoriasis was once considered as a mere localized cutaneous disease demonstrating well-demarcated erythematous plaque with silvery scales. However, intensive studies changed the main concept of the disease, which led to a new perspective of psoriasis as an immune-mediated disease with systemic inflammation and associated comorbidities, including inflammatory bowel disease, metabolic syndrome, cardiovascular disease, psoriatic arthritis, chronic kidney disease and mood disorders.[4],[5],[15],[16],[17],[18] Retrospective research from a large academic setting revealed that 73% of patients with psoriasis have at least one comorbidity.[19] Another study revealed higher prevalence of selected comorbidities of interest in patients with psoriasis compared with control patients (odds ratio, 1.68; 95% confidence interval, 1.66–1.69), which was observed in all age groups.[7] Therefore, comprehensive management of psoriasis and its comorbidities appears to be important.[20]
Although inflammatory bowel disease is one of the representative comorbidities of psoriasis, the epidemiology of this association remains poorly defined.[5] In Korea, even the nationwide prevalence of inflammatory bowel disease itself was not known, and only the prevalence in a localized district of Seoul (Songpa-Kangdong area, 1986–2005) had been reported.[21] In this previous report, as of December 31, 2005, overall age- and sex-adjusted prevalence was 11.24/100,000 inhabitants (0.01%) for Crohn's disease and 30.87/100,000 inhabitants (0.03%) for ulcerative colitis.[21] Other data from two teaching hospitals located in Seoul (1990–2012) revealed higher prevalence of Crohn's disease (0.043%) and ulcerative colitis (0.054%) among patients with psoriasis (n = 9322)[22] compared with the general population[21]; however, the number of included patients was much too small (n = 4 for Crohn's disease; n = 5 for ulcerative colitis) to draw a definite conclusion. The present study demonstrated that the nationwide prevalence of Crohn's disease and ulcerative colitis were 0.03–0.04% (49–71/100,000) and 0.06–0.07% (124–138/100,000), respectively, from 2011 through 2015, which increased with time. This pattern is compatible with a previous systematic review revealing increased incidence and prevalence of inflammatory bowel disease with time and in different areas around the world.[23] Notably, nationwide epidemiologic data of inflammatory bowel disease concurrent with psoriasis were scarce, but the present study identified the volume of patients with both diseases.
Previous epidemiologic studies demonstrated conflicting results regarding the association between inflammatory bowel disease and psoriasis. In particular, research from Taiwan using the National Health Insurance claims database reported that patients with psoriasis demonstrated lower risk of prevalent Crohn's disease compared with controls (adjusted risk ratio, 0.66; 95% confidence interval, 0.48–0.91).[11] They postulated that the discrepancies with previous studies were due to different ethnic backgrounds leading to differences in shared genetic susceptibility loci between psoriasis and inflammatory bowel disease. However, the present study does not suggest racial differences regarding the association between inflammatory bowel disease and psoriasis and demonstrated results consistent with previous studies from Western countries. In addition, a previous study in Korea demonstrated that patients with inflammatory bowel disease had higher risk of inflammatory skin disease, including psoriasis, which supports our findings in a way and helps to describe a bidirectional relationship between psoriasis and inflammatory bowel disease.[24]
Whereas previous studies mostly included adult patients[6],[8],[12],[13] or did not perform subgroup analyses for underage patients,[7],[9],[11] we further analyzed patients younger than 19 years of age. In 2015, patients with psoriasis younger than 19 years of age demonstrated the highest odds of prevalent Crohn's disease and ulcerative colitis, especially Crohn's disease, compared with the general population of the same age. Approximately one-fourth of patients with inflammatory bowel disease have onset before 20 years of age.[25] Additionally, pediatric patients with inflammatory bowel disease more frequently present with nonclassical symptoms[26] and extraintestinal manifestations, including anemia, uveitis, autoimmune hepatitis, arthritis, growth failure, osteopenia, pyoderma gangrenosum and erythema nodosum.[26],[27] Extraintestinal manifestations are more common in pediatric patients with Crohn's disease (15–25%) than ulcerative colitis (2–16%).[28],[29] These characteristics may explain the strong association between psoriasis and inflammatory bowel disease, especially Crohn's disease, in the younger age group.[26],[30] Although a thorough literature search by a psoriasis expertise panel found only a limited number of studies on pediatric psoriasis comorbidities, they recommended formal gastrointestinal evaluation in pediatric patients with psoriasis and unexplained growth retardation.[31] It is noteworthy that our study added adequate evidence. Furthermore, patients with concurrent psoriasis and inflammatory bowel disease in this age group may require a different treatment strategy to suppress autoimmunity, employing immune modulators or biologics, rather than conventional treatment.
Severe psoriasis demonstrated a higher association with concurrent inflammatory bowel disease (both Crohn's disease and ulcerative colitis) compared with mild psoriasis. In the present study, severe psoriasis was defined by the use of systemic psoriasis treatments or phototherapy. As some systemic antipsoriatic agents (biologics, cyclosporine, methotrexate) are also used for inflammatory bowel disease, the association between severe psoriasis and inflammatory bowel disease can be overestimated. Among biologics, infliximab and adalimumab are generally used to treat inflammatory bowel disease in Korea. Other overlapping agents, including cyclosporine and methotrexate, are scarcely used to treat inflammatory bowel disease in Korea. Therefore, we performed an additional analysis after excluding patients who had been treated with infliximab and adalimumab. As a result, the relationship between severe psoriasis and Crohn's disease was lost, whereas the association with ulcerative colitis was maintained. This may simply be because clinicians more frequently prescribe infliximab/adalimumab for Crohn's disease than for ulcerative colitis, or because patients with psoriasis and concurrent Crohn's disease truly had more severe psoriasis requiring treatment with infliximab/adalimumab, rather than only ulcerative colitis has true association.
Epidemiologic studies using a randomized psoriasis population or specific cohort may provide limited information. On the contrary, a very large insurance claims database provides more generalizable information on the prevalence of comorbidities. South Korea has been adopting a single mandatory national health insurance system and all types of healthcare institutions must submit health claims data to the Health Insurance Review and Assessment Service in order to receive reimbursement. In this way, the Health Insurance Review and Assessment Service has accumulated a vast amount of medical records since 2002 and has become one of world's largest medical databases. For example, the Health Insurance Review and Assessment Service gathered 1.3 billion health claims data from the entire Korean population in 2011.[32],[33],[34],[35] Similarly, the present study included the entire Korean population (approximately 50 million people) and can reflect real-world patients. Because a cross-sectional study using a health claims database usually targets the population of a specific country, further studies including a global population or a systematic review of various research is desired.
Our study has several limitations. First, we did not evaluate lifestyle factors, such as smoking, diet, alcohol intake or body mass index. Also, we had limited data for doing adjustment and multivariate analysis. Second, we did not evaluate causal or temporal relationships due to the cross-sectional design. Third, detection or misclassification bias is possible. In spite of several limitations, this was a large-scale population-based study, including the entire Korean population, regarding prevalence of inflammatory bowel disease in patients with psoriasis compared with the general population. The present study suggests that prevalence of inflammatory bowel disease in patients with psoriasis significantly increased with time and patients with psoriasis revealed higher prevalence of inflammatory bowel disease compared with the general population. In addition, patients with psoriasis younger than 19 years of age demonstrated the highest risk of inflammatory bowel disease, especially Crohn's disease. Further work on these associations may enable us to better manage both inflammatory bowel disease and psoriasis and to reduce the burden.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form, the patients have given their consent for their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
| 1. |
Oh CJ, Das KM, Gottlieb AB. Treatment with anti-tumor necrosis factor alpha (TNF-alpha) monoclonal antibody dramatically decreases the clinical activity of psoriasis lesions. J Am Acad Dermatol 2000;42:829-30.
[Google Scholar]
|
| 2. |
Targan SR, Hanauer SB, van Deventer SJ, Mayer L, Present DH, Braakman T, et al. A short-term study of chimeric monoclonal antibody cA2 to tumor necrosis factor alpha for Crohn's disease. Crohn's disease cA2 study group. N Engl J Med 1997;337:1029-35.
[Google Scholar]
|
| 3. |
Najarian DJ, Gottlieb AB. Connections between psoriasis and Crohn's disease. J Am Acad Dermatol 2003;48:805-21.
[Google Scholar]
|
| 4. |
Rivas Bejarano JJ, Valdecantos WC. Psoriasis as autoinflammatory disease. Dermatol Clin 2013;31:445-60.
[Google Scholar]
|
| 5. |
Takeshita J, Grewal S, Langan SM, Mehta NN, Ogdie A, Van Voorhees AS, et al. Psoriasis and comorbid diseases: Epidemiology. J Am Acad Dermatol 2017;76:377-90.
[Google Scholar]
|
| 6. |
Makredes M, Robinson D Jr., Bala M, Kimball AB. The burden of autoimmune disease: A comparison of prevalence ratios in patients with psoriatic arthritis and psoriasis. J Am Acad Dermatol 2009;61:405-10.
[Google Scholar]
|
| 7. |
Augustin M, Reich K, Glaeske G, Schaefer I, Radtke M. Co-morbidity and age-related prevalence of psoriasis: Analysis of health insurance data in Germany. Acta Derm Venereol 2010;90:147-51.
[Google Scholar]
|
| 8. |
Cohen AD, Dreiher J, Birkenfeld S. Psoriasis associated with ulcerative colitis and Crohn's disease. J Eur Acad Dermatol Venereol 2009;23:561-5.
[Google Scholar]
|
| 9. |
Wu JJ, Nguyen TU, Poon KY, Herrinton LJ. The association of psoriasis with autoimmune diseases. J Am Acad Dermatol 2012;67:924-30.
[Google Scholar]
|
| 10. |
Augustin M, Glaeske G, Radtke MA, Christophers E, Reich K, Schäfer I, et al. Epidemiology and comorbidity of psoriasis in children. Br J Dermatol 2010;162:633-6.
[Google Scholar]
|
| 11. |
Tsai TF, Wang TS, Hung ST, Tsai PI, Schenkel B, Zhang M, et al. Epidemiology and comorbidities of psoriasis patients in a national database in Taiwan. J Dermatol Sci 2011;63:40-6.
[Google Scholar]
|
| 12. |
Egeberg A, Mallbris L, Warren RB, Bachelez H, Gislason GH, Hansen PR, et al. Association between psoriasis and inflammatory bowel disease: A Danish nationwide cohort study. Br J Dermatol 2016;175:487-92.
[Google Scholar]
|
| 13. |
Li WQ, Han JL, Chan AT, Qureshi AA. Psoriasis, psoriatic arthritis and increased risk of incident Crohn's disease in US women. Ann Rheum Dis 2013;72:1200-5.
[Google Scholar]
|
| 14. |
Takeshita J, Grewal S, Langan SM, Mehta NN, Ogdie A, Van Voorhees AS, et al. Psoriasis and comorbid diseases: Implications for management. J Am Acad Dermatol 2017;76:393-403.
[Google Scholar]
|
| 15. |
Christophers E. Comorbidities in psoriasis. Clin Dermatol 2007;25:529-34.
[Google Scholar]
|
| 16. |
Sundarrajan S, Arumugam M. Comorbidities of psoriasis-exploring the links by network approach. PLoS One 2016;11:e0149175.
[Google Scholar]
|
| 17. |
Gottlieb AB, Chao C, Dann F. Psoriasis comorbidities. J Dermatolog Treat 2008;19:5-21.
[Google Scholar]
|
| 18. |
Machado-Pinto J, Diniz Mdos S, Bavoso NC. Psoriasis: New comorbidities. An Bras Dermatol 2016;91:8-14.
[Google Scholar]
|
| 19. |
Pearce DJ, Morrison AE, Higgins KB, Crane MM, Balkrishnan R, Fleischer AB Jr., et al. The comorbid state of psoriasis patients in a university dermatology practice. J Dermatolog Treat 2005;16:319-23.
[Google Scholar]
|
| 20. |
Shah K, Mellars L, Changolkar A, Feldman SR. Real-world burden of comorbidities in US patients with psoriasis. J Am Acad Dermatol 2017;77:287-92.e4.
[Google Scholar]
|
| 21. |
Yang SK, Yun S, Kim JH, Park JY, Kim HY, Kim YH, et al. Epidemiology of inflammatory bowel disease in the Songpa-Kangdong district, Seoul, Korea, 1986-2005: A KASID study. Inflamm Bowel Dis 2008;14:542-9.
[Google Scholar]
|
| 22. |
Park HS, Koh SJ, Park GY, Lee DH, Yoon HS, Youn JI, et al. Psoriasis concurrent with inflammatory bowel disease. J Eur Acad Dermatol Venereol 2014;28:1436-41.
[Google Scholar]
|
| 23. |
Molodecky NA, Soon IS, Rabi DM, Ghali WA, Ferris M, Chernoff G, et al. Increasing incidence and prevalence of the inflammatory bowel diseases with time, based on systematic review. Gastroenterology 2012;142:46-5.e42.
[Google Scholar]
|
| 24. |
Kim M, Choi KH, Hwang SW, Lee YB, Park HJ, Bae JM, et al. Inflammatory bowel disease is associated with an increased risk of inflammatory skin diseases: A population-based cross-sectional study. J Am Acad Dermatol 2017;76:40-8.
[Google Scholar]
|
| 25. |
Baldassano RN, Piccoli DA. Inflammatory bowel disease in pediatric and adolescent patients. Gastroenterol Clin North Am 1999;28:445-58.
[Google Scholar]
|
| 26. |
Rosen MJ, Dhawan A, Saeed SA. Inflammatory bowel disease in children and adolescents. JAMA Pediatr 2015;169:1053-60.
[Google Scholar]
|
| 27. |
Griffiths AM. Specificities of inflammatory bowel disease in childhood. Best Pract Res Clin Gastroenterol 2004;18:509-23.
[Google Scholar]
|
| 28. |
Mamula P, Markowitz JE, Baldassano RN. Inflammatory bowel disease in early childhood and adolescence: Special considerations. Gastroenterol Clin North Am 2003;32:967-95, viii.
[Google Scholar]
|
| 29. |
Fish D, Kugathasan S. Inflammatory bowel disease. Adolesc Med Clin 2004;15:67-90, ix.
[Google Scholar]
|
| 30. |
Diefenbach KA, Breuer CK. Pediatric inflammatory bowel disease. World J Gastroenterol 2006;12:3204-12.
[Google Scholar]
|
| 31. |
Osier E, Wang AS, Tollefson MM, Cordoro KM, Daniels SR, Eichenfield A, et al. Pediatric psoriasis comorbidity screening guidelines. JAMA Dermatol 2017;153:698-704.
[Google Scholar]
|
| 32. |
Eun SJ, Jang S, Lee JY, Do YK, Jo SJ. High rate of systemic corticosteroid prescription among outpatient visits for psoriasis: A population-based epidemiological study using the Korean National Health Insurance database. J Dermatol 2017;44:1027-32.
[Google Scholar]
|
| 33. |
Park SY, Lee JY, Lim NG, Hong YH. Incidence and prevalence of myasthenia gravis in Korea: A population-based study using the national health insurance claims database. J Clin Neurol 2016;12:340-4.
[Google Scholar]
|
| 34. |
Yang MS, Lee JY, Kim J, Kim GW, Kim BK, Kim JY, et al. Incidence of Stevens-Johnson syndrome and toxic epidermal necrolysis: A nationwide population-based study using national health insurance database in Korea. PLoS One 2016;11:e0165933.
[Google Scholar]
|
| 35. |
Park SB, Kim J, Jeong JH, Lee JK, Chin DK, Chung CK, et al. Prevalence and incidence of osteoporosis and osteoporotic vertebral fracture in Korea: Nationwide epidemiological study focusing on differences in socioeconomic status. Spine (Phila Pa 1976) 2016;41:328-36.
[Google Scholar]
|
Fulltext Views
4,931
PDF downloads
2,122





