Translate this page into:
Review of the reasons for and effectiveness of switching biologics for psoriasis treatment in Korea
Corresponding author: Dr. Byungsoo Kim, Department of Dermatology, Pusan National University Hospital, 305 Gudeok-ro, Seo-gu, Busan, Republic of Korea. dockbs@pusan.ac.kr
-
Received: ,
Accepted: ,
How to cite this article: Shin J-O, Shin BS, Bae K-N, Shin K, Kim H-S, Ko H-C et al. Review of the reasons for and effectiveness of switching biologics for psoriasis treatment in Korea. Indian J Dermatol Venereol Leprol 2023;89:928
Abstract
Background
Switching of biologics in patients has become common in clinical practice.
Objectives
This study investigated the reasons for and effectiveness of switching biologic agents during the treatment of psoriasis.
Methods
We retrospectively reviewed patients with psoriasis who were treated with biologics at Pusan National University Hospital and Chosun University Hospital from March 2012 to June 2020. We assessed their demographics and treatment characteristics (reasons for switching biologics and efficacy of the first- and second biologic agents).
Results
Of the 162 psoriatic patients treated with biologic agents for more than 52 weeks, 35 required a switch to another biologic agent. The reasons for switching biologic agents were inefficacy (n = 30), adverse events (n = 2) and others (n = 3). The mean psoriasis area and severity index (PASI) score was 12.1 at the start of the second biologic and 3.4 at 14–16 weeks later. Patients were more likely to switch to another biologic agent when they exhibited a high initial psoriasis area and severity index score and concomitant psoriatic arthritis.
Limitations
As a retrospective study, there were some limitations such as lack of a placebo control group and the time point of 14–16 weeks being somewhat early to judge the effect of the biologics.
Conclusions
The most common reason for switching biologic agents in Korea was treatment inefficacy, especially secondary failure. Despite the inefficacy of previous biologic agents, switching to a different agent may be an efficacious approach.
Keywords
Biologics
psoriasis
treatment
Plain Language Summary
Biologics have been used for the treatment of psoriasis in patients who do not response to conventional treatments. However, in clinical practice, switching of biologics has become common. We investigate the reasons and efficacy of switching biologics in Korean patients with moderate and severe plaque psoriasis. The most common reason for switching biologics in Korea was treatment inefficacy, especially secondary failure. Despite the inefficacy of previous biologics, switching to a different agent may be an efficacious treatment option.
Introduction
Psoriasis is an inflammatory skin disease characterised by papules and plaques with silvery white scales. Patients with psoriasis are less satisfied with their lives due to the condition,1 and they are more likely to have accompanying systemic diseases such as cardiovascular disease and metabolic syndrome compared to people without psoriasis.2,3 In recent decades, the immunological aetiology of psoriasis has been revealed, and various biologic agents have been developed to act on these immunological pathways.
In Korea, since 2011, biologic agents have been used for the treatment of psoriasis in patients who do not respond to conventional treatments. However, biologic agents do not consistently exhibit equivalent therapeutic effects in all patients, and some show insufficient treatment responses or adverse reactions. In these cases, switching biologic agents may be considered. However, few studies have reported the reasons and efficacy of switching biologic agents in psoriasis.
This study investigated the reasons and efficacy of switching biologic agents in Korean patients with moderate and severe plaque psoriasis.
Methods
Study population
This study included patients aged 18 years or older who visited Pusan National University Hospital and Chosun University Hospital department of dermatology between March 2012 and June 2020, who were diagnosed with psoriasis and treated with biological drugs for 52 weeks or more by satisfying the following inclusion criteria:
Plaque psoriasis lasting for more than 6 months
Psoriasis area and severity index of 10 or higher
Involvement of more than 10% of the total body surface area
Insufficient response to acitretin, cyclosporine, methotrexate and phototherapy, or conventional treatment cannot be continued due to side effects
Study design
Demographics
Data on patient age, sex, body mass index (BMI), disease duration, psoriatic arthritis, nail involvement, usage of systemic drugs and psoriasis area and severity index scores before using biologics were obtained from their medical records and were analysed.
Therapeutic efficacy of the biologic agents
The therapeutic effects of the first and second biologic agents were analysed in patients who switched to other biologic agents. To evaluate the therapeutic effect of the biologic agents, the psoriasis area and severity index scores before administration of the biologic agent (week 0) and 14–16 weeks after administration were evaluated to analyse the rate of decrease. PASI 50, 75, 90 and 100 were defined as cases where the psoriasis area and severity index score improved by at least 50, 75, 90 and 100%, respectively, or more after 14–16 weeks of biologics administration compared to those before administration. The durations of administration of the biologic agents were also evaluated.
Reasons for switching to a different biologic agent
The reasons for switching to another biologic agent are classified as follows:
Primary failure, defined as a failure to achieve PASI 75 at 14–16 weeks after biologic administration.
Secondary failure, defined as cases in which PASI 75 was achieved at the initial evaluation after biological agent administration, but the effect of the biological agent decreased over time and the treatment response of PASI 75 was not maintained.
Adverse events
Others
Associated clinical factors in the biologics-continued and -discontinued groups
Patients who were administered biologics continuously and those who stopped biological agent treatment were compared. Several clinical factors (age, sex, BMI, PASI score before use of biological agents, disease duration, psoriatic arthritis and nail involvement) were compared between the continued and discontinued groups.
Adverse events
Medical history and blood test results were assessed to investigate the side effects related to drug administration during treatment.
The study was approved by the Institutional Review Board of Pusan National University Hospital (IRB No.2102-011-100), and all patients or their parents signed informed consent forms for the study.
All statistical analyses were performed using IBM SPSS Statistics for Windows, version 25.0 (IBM Corp., NY, USA). For comparisons of data between the two groups, Mann–Whitney U or Fisher’s exact tests were performed. Statistical significance was defined as P < 0.05.
Results
Demographics and clinical features
From March 2012 to June 2020, among adults aged 18 years or older who visited the Department of Dermatology at Pusan National University Hospital and Chosun University Hospital, a total of 162 patients diagnosed with psoriasis were treated with biologics for 52 weeks or more. Table 1 shows the demographic and clinical features of all the patients. Of the 162 patients, 108 (66.7%) were men, and the male to female ratio was 2:1. The age distribution ranged from 18 to 75 years, with an average age of 42.3 ± 12.5 years. The biologics administered were ustekinumab (n = 91), secukinumab (n = 59) and adalimumab (n = 12).
BMI: body mass index, PASI: Psoriasis Area and Severity Index, SD: Standard Deviation Data are presented as n (%) or mean ± SD, as appropriate.
Demographics
Total
Ustekinumab
Secukinumab
Adalimumab
No. of patients
162
91
59
12
Age, years
42.3 ± 12.5
42.2 ± 11.3
43.3 ± 12.5
38.3 ± 19.4
Sex
Male, n (%)
108 (66.7)
62 (68.1)
41 (69.5)
5 (41.7)
Female, n (%)
54 (33.3)
29 (31.9)
18 (30.5)
7 (58.3)
BMI, kg/m2
23.6 ± 3.3
23.7 ± 3.4
23.5 ± 3.2
23.6 ± 3.4
Psoriatic arthritis, n (%)
45 (27.8)
21 (23.1)
13 (22.0)
11 (91.7)
Nail involvement, n (%)
47 (29.0)
27 (23.1)
14 (23.7)
6 (50.0)
Disease duration, months
167.9 ± 111.4
181.3 ± 112.1
158.4 ± 111.7
112.0 ± 86.7
Conventional treatment before biologics initiation, n (%)
Acitretin
21 (13.0)
13 (14.3)
4 (6.8)
4 (33.3)
Cyclosporin
91 (56.2)
54 (59.3)
30 (50.8)
7 (58.3)
Methotrexate
119 (73.4)
72 (79.1)
36 (61.0)
11 (91.7)
PASI at baseline
16.2 ± 6.1
16.5 ± 6.5
16.9 ± 5.3
10.8 ± 3.5
Duration of the first biologic treatment, weeks
133.3 ± 88.6
170.7 ± 99.3
87.9 ± 33.0
73.2 ± 42.2
The average psoriasis area and severity index score before biologics administration was 16.2 ± 6.1, and the average body mass index of the patients was 25.7 ± 3.6 kg/m.2 Prior systemic treatment was performed in all patients before starting the biologics treatment. Methotrexate was most commonly administered (119 patients, 73.4%), followed by cyclosporine (91, 56.2%) and acitretin (21, 13.0%).
The average period from the time of diagnosis of psoriasis to the administration of biologics was 167.9 ± 111.4 months. At the time of biologics administration, 45 patients (27.8%) had psoriatic arthritis and 47 patients (29.0%) had nail involvement.
Of the 162 patients, 118 (72.8%) continued to use the first biologic agent, 44 (27.2%) discontinued the first biologic agent and 35 (21.6%) used the second biologic agent.
Therapeutic efficacy and reasons for switching biologics
Among the 35 patients who changed biologic agents, the psoriasis area and severity index scores before the administration of the first agent ranged from 10.2 to 32.5 (average, 19.9). The average psoriasis area and severity index score after 14–16 weeks of administration was 4.0, which indicated a statistically significant decrease (P < 0.05). The average duration of administration of the first biologic agent was 133.3 ± 88.6 weeks. The psoriasis area and severity index scores before administration of the second biologic agent ranged from 1.3 to 19.8 (average, 12.1). The average psoriasis area and severity index score after 14–16 weeks of administration was 3.4, which again indicated a statistically significant decrease (P < 0.05) [Figure 1]. The psoriasis area and severity index scores after 14–16 weeks of administration of each second agent are described in [Figure 2]. The proportions of patients who achieved PASI 75, PASI 90 and PASI 100 at 14–16 weeks after administration of the first biologic agent were 57.1, 17.1 and 2.9%, respectively, and 51.4, 14.3 and 2.9%, respectively, after administration of the second biologic agent.
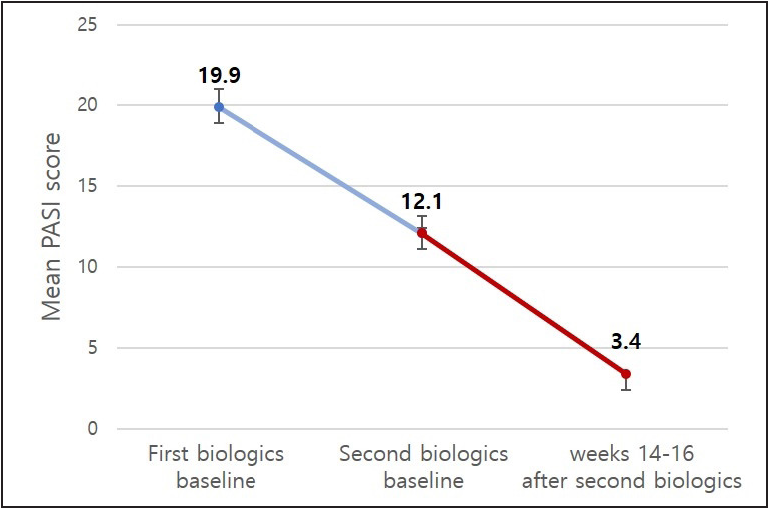
- Mean PASI score at baseline of the first and second biologics and after 14–16 weeks of using the second biologics among patients who switched biologic agents (n = 35). PASI: Psoriasis Area and Severity Index
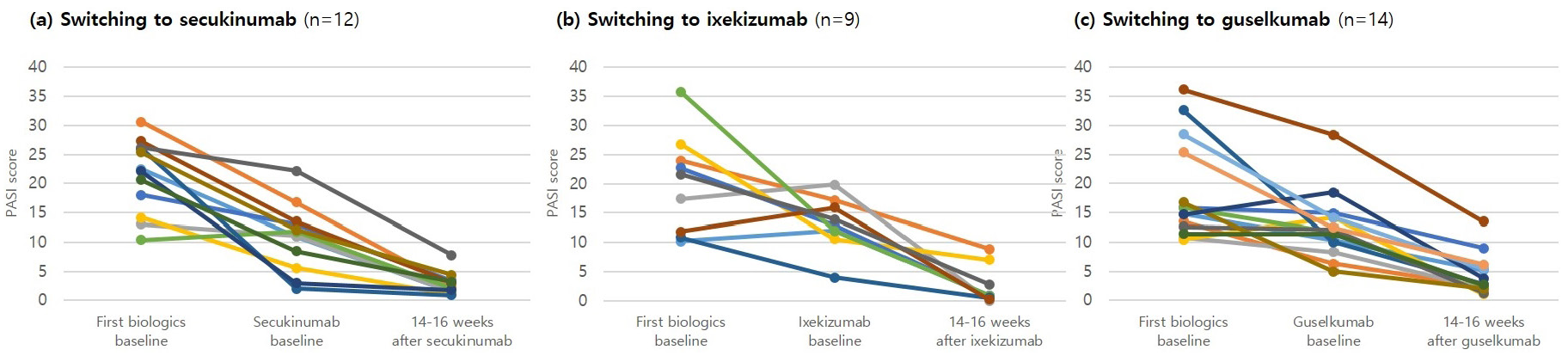
- PASI score at baseline of the first and second biologics and after 14–16 weeks of using the second biologics in each patients who switched biologics. Second biologics as follows: (a) secukinumab (n = 12), (b) ixekizumab (n = 9), (c) guselkumab (n = 14)
Table 2 shows the number of patients and their reasons for switching biologic agent. Of the 35 patients who changed biologics, 28 (80.0%) did so due to secondary failure. Two (5.7%) patients had primary failure, and two (5.7%) patients changed biologics due to adverse events.
Data are presented as n (%) or mean ± SD, as appropriate.
Demographics
Total
Ustekinumab
Secukinumab
Adalimumab
No. of patients
162
91
59
12
No. of continuously treated patients, n (%)
118 (72.8)
64 (70.3)
46 (78.0)
10 (83.3)
No. of discontinued patients, n (%)
44 (27.2)
27 (29.7)
13 (22.0)
2 (16.7)
No. of switched patients, n (%)
35 (21.6)
23 (25.3)
10 (16.9)
2 (16.7)
To secukinumab
12
10
-
2
To ixekizumab
9
5
4
0
To guselkumab
14
8
6
0
Associated clinical factors in the biologic-continued and -discontinued group
Table 3 compares the clinical factors (age, sex, BMI, PASI score before use of the biologics, disease duration, presence of psoriatic arthritis and nail involvement) between the group of patients who continuously received biologics and the group who stopped their first biologic agent.
Data are presented as n (%)
Total
Ustekinumab
Secukinumab
Adalimumab
No. of patients
35
23
10
2
Inefficacy
30 (85.7)
19
10
1
Primary failure*
2 (5.7)
1
0
1
Secondary failure**
28 (80.0)
18
10
0
Adverse events***
2 (5.7)
1
0
1
Others¶
3 (8.6)
3
0
0
First, the average psoriasis area and severity index score before the use of the biologics was significantly higher in the group of patients who discontinued the biologics compared to that in the group that was continuously administered biologics (19.3 ± 7.0 vs. 15.1 ± 5.3, P = 0.001).
Second, 19 patients (43.2%) in the group that discontinued the first biologic therapy had psoriatic arthritis, compared to 28 patients (23.7%) in the group with continued biologic administration (P = 0.015). There were no statistically significant differences in age, sex, BMI, disease duration or nail involvement between the two groups.
Adverse events
A total of 16 patients (9.9%) experienced adverse reactions after the first use of biologics (six with injection site reactions such as erythema and pain at the injection site, three with upper respiratory infections, two with urticaria, two with folliculitis, one with headache, one with drug-induced lupus and one with elevated levels of aspartate aminotransferase and alanine aminotransferase in liver function tests).
Two patients discontinued treatment with the first biologic agent due to the above reactions (one with injection site reaction and one with drug-induced lupus). After the administration of the second biologic, three patients (8.6%) experienced adverse reactions (two with injection-site reactions and one with upper respiratory tract infection). However, no patient discontinued treatment with the second biologic agent due to the above reactions, and there were no serious side effects that posed serious threat to life or cases that required hospitalization.
Discussion
Despite their benefits, some patients discontinue biologic treatment due to insufficient therapeutic effects or adverse reactions. Waren4 reported that one-third and nearly one-half of patients stopped treatment within 1 year and after 3 years, respectively, following the first use of biologics. In this study, 44 (27.2%) of the 162 patients discontinued their first biologics, 35 (21.6%) of whom switched to other biologic agents.
In this study, among the 35 patients who switched biologic agents, 79.9%, achieved psoriasis area and severity index reduction after 14–16 weeks of administration of the first biologic agent, and 72.1% achieved psoriasis area and severity index reduction after the administration of the second biologic agent. Compared to the first biologic agent, the slightly lower rate of psoriasis area and severity index reduction after the use of the second biologic agent is due to the lower psoriasis area and severity index score before the use of the second biologics than before the use of the first agent. However, patients who switched to a second biologic agent also showed significant decreases in the psoriasis area and severity index score (P < 0.05). In addition, the rates of achievement of PASI 75, PASI 90 and PASI 100 were similar after administration of the first and second biologic agents. This suggests that switching to other biologic agents may be a treatment option that could provide significant clinical improvement even if the previous biologic agents were insufficiently effective or if the medication could not be continued due to side effects.
In addition, to identify the clinical factors related to biologics discontinuation, the group of patients that continued biologic use and the group that discontinued the first biologics were compared. Only patients with high psoriasis area and severity index scores and psoriatic arthritis showed significant differences before biologics use. The clinical factors associated with the discontinuation of the first biologic agent may be related to a poor response. Because it is known that the higher the severity of psoriasis, the lower the response to biological agents,5 the association between high pre-treatment psoriasis area and severity index score and biologics discontinuation was assessed. Disease severity is also associated with the presence of psoriatic arthritis.6,7 Therefore, patients with high psoriasis area and severity index scores or concomitant psoriatic arthritis before starting biologics tend to switch and the commonest reason for switching is secondary failure.
The most common reason for switching to biologics in this study too was secondary failure. Twenty-eight (80.0%) patients switched biologics due to a decrease in the efficacy of the initial biologic agent over time. The decrease in therapeutic effect is partly due to the generation of anti-drug antibodies against that biologic agent.8 Two patients (5.7%) switched due to adverse reactions (suspected injection site reaction and drug-induced lupus). Compared to previous reports,9,10 most of the patients in this study switched biologics due to secondary failure, and the proportion of patients who changed biologics due to primary failure or adverse reactions was relatively low.
In this study, 21.6% of patients changed biologic agents, a rate similar to those reported previously (18.5–34%).9,10 The efficacy of the switched biologics, that is, the reduction rate of the average psoriasis area and severity index score at 14–16 weeks after replacement, was 72.1%, which was higher than the previously reported 53.3%.9 In addition, the average duration of administration of the first biologics was 133.3 weeks, which was longer than that previously reported (47.6–94.19,10 weeks). These differences may be due to differences in demographic characteristics between studies. Because the participants in this study had a lower body mass index compared to those in the previous study10 (23.6 kg/m2 vs. 27.1 kg/m2), they had a higher drug dose to body weight, even if they were administered the same dose of biologics; thus, the biologics showed a better effect. Previous studies on biological drugs such as ustekinumab,11 secukinumab12 and ixekizumab13 in Koreans have shown their superior therapeutic effects compared to those in foreign clinical studies. However, a study with a larger population and longer follow-up period is needed to confirm this finding.
One of the limitations of this study was that the total number of patients was smaller due to problems such as insurance coverage standards for biologics as well as high prices. In addition, as a retrospective study, there were limitations such as the absence of a placebo control group and that the time point of 14–16 weeks was somewhat early to judge the effect of the biologics. Another limitation is that the various biologics were approved during different times during the study period.
In conclusion, in this study, 21.6% of patients with moderate to severe plaque psoriasis who were administered biological agents replaced their initial agent. The main reason for replacement was a decreased therapeutic effect of the first biologic agent over time. Even if the previous biologics were insufficiently effective or the drug could not be continued due to side effects, significant clinical improvement was observed after replacement of the biologic agent. We suggest that switching to another biologic agent may be an effective method in the treatment of patients with plaque psoriasis who lack a response to previous treatment or cannot continue administration due to adverse reactions.
The group in which ustekinumab was administered as the first drug
The group in which secukinumab was administered as the first drug
The group in which adalimumab was administered as the first drug
All subjects
Data are presented as n (%) or mean ± SD, as appropriate.
Ustekinumab
Continued
Discontinued
P
No. of patients
64
27
Age, years
41.8 ± 11.5
43.1 ± 11.0
0.620
Sex, male (%)
44 (68.8)
18 (66.7)
0.038
BMI, kg/m2
23.6 ± 3.6
23.9 ± 2.9
0.691
PASI baseline
15.2 ± 5.8
19.7 ± 7.0
0.005
Disease duration, months
179.6 ± 111.2
185.3 ± 116.5
0.826
Psoriatic arthritis (%)
9 (14.1)
13 (48.1)
0.001
Nail involvement (%)
14 (21.9)
13 (48.1)
0.012
Secukinumab
Continued
Discontinued
P
No. of patients
44
15
Age, years
42.1 ± 11.8
46.7 ± 14.0
0.225
Sex, male (%)
31 (70.5)
10 (66.7)
0.758
BMI, kg/m2
23.5 ± 3.3
23.6 ± 2.9
0.885
PASI baseline
16.0 ± 4.3
19.4 ± 7.1
0.098
Disease duration, months
153.0 ± 108.1
174.4 ± 124.1
0.526
Psoriatic arthritis (%)
10 (22.7)
4 (26.7)
0.738
Nail involvement (%)
4 (9.1)
10 (66.7)
0.738
Adalimumab
Continued
Discontinued
P
No. of patients
10
2
Age, years
38.1 ± 21.1
39.5 ± 23.0
0.931
Sex, male (%)
5 (50.0)
0 (0.0)
BMI, kg/m2
23.7 ± 3.6
23.3 ± 2.1
0.868
PASI baseline
10.3 ± 3.7
13.0 ± 1.4
0.342
Disease duration, months
25.9 ± 3.1
23.5 ± 2.1
0.324
Psoriatic arthritis (%)
9 (90.0)
2 (100.0)
1.000
Nail involvement (%)
6 (60.0)
0 (0.0)
0.455
Total
Continued
Discontinued
P
No. of patients
118
44
Age, years
41.6 ± 12.6
44.2 ± 12.0
0.248
Sex, male (%)
80 (67.8)
28 (63.6)
0.617
BMI, kg/m2
23.6 ± 3.5
23.8 ± 2.8
0.713
PASI baseline
15.1 ± 5.3
19.3 ± 7.0
0.001
Disease duration, months
164.0 ± 109.8
178.1 ± 116.1
0.477
Psoriatic arthritis (%)
28 (23.7)
19 (43.2)
0.015
Nail involvement (%)
30 (25.4)
17 (38.6)
0.099
Declaration of patient consent
Patient consent was not required as their identity is not disclosed or compromised.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- Psoriasis causes as much disability as other major medical diseases. J Am Acad Dermatol. 1999;41:401-7.
- [CrossRef] [PubMed] [Google Scholar]
- Psoriasis and comorbid diseases: Implications for management. J Am Acad Dermatol. 2017;76:393-403.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Psoriasis as a systemic disease. Clin Dermatol. 2014;32:343-50.
- [CrossRef] [PubMed] [Google Scholar]
- Differential drug survival of biologic therapies for the treatment of psoriasis: A prospective observational cohort study from the British Association of Dermatologists Biologic Interventions Register (BADBIR) J Invest Dermatol. 2015;135:2632-40.
- [CrossRef] [PubMed] [Google Scholar]
- Plaque thickness and morphology in psoriasis vulgaris associated with therapeutic response. Br J Dermatol. 2009;160:1083-89.
- [CrossRef] [PubMed] [Google Scholar]
- Efficacy and safety of ustekinumab, a human interleukin-12/23 monoclonal antibody, in patients with psoriasis: 52-week results from a randomised, double-blind, placebo-controlled trial (PHOENIX 2) Lancet. 2008;371:1675-84.
- [CrossRef] [PubMed] [Google Scholar]
- Etanercept in severe, recalcitrant psoriasis: Clinical response, safety profile and predictors of response based on a single institution’s experience. J Eur Acad Dermatol Venereol. 2009;23:979-82.
- [CrossRef] [PubMed] [Google Scholar]
- Sustainability and switching of biologics for psoriasis and psoriatic arthritis at Fukuoka University Psoriasis Registry. J Dermatol. 2019;46:389-98.
- [CrossRef] [PubMed] [Google Scholar]
- Switching of biologics in psoriasis: Reasons and results. J Dermatol. 2017;44:1015-19.
- [CrossRef] [PubMed] [Google Scholar]
- Switching biologics in the treatment of psoriasis: A multicenter experience. Dermatology. 2021;237:22-30.
- [CrossRef] [PubMed] [Google Scholar]
- Efficacy and safety of ustekinumab in the treatment of moderate to severe psoriasis in Korea. Korean J Dermatol. 2015;53:617-22.
- [Google Scholar]
- Efficacy and safety of secukinumab for the treatment of moderate to severe psoriasis in Korea. Korean J Dermatol. 2019;57:9-14.
- [Google Scholar]
- Efficacy and safety of ixekizumab for the treatment of moderate to severe psoriasis in Korea. Korean J Dermatol. 2020;58:389-96.
- [Google Scholar]






