Translate this page into:
Serum macrophage migration inhibitory factor levels in leprosy patients with erythema nodosum leprosum
2 Department of Dermatology, Postgraduate Institute of Medical Education and Research, Chandigarh, India
Correspondence Address:
Seema Chhabra
Department of Immunopathology, Room No. 29, 4th Floor, Research Block-A, Postgraduate Institute of Medical Education and Research, Sector-12, Chandigarh
India
| How to cite this article: Bansal F, Narang T, Dogra S, Vinay K, Chhabra S. Serum macrophage migration inhibitory factor levels in leprosy patients with erythema nodosum leprosum. Indian J Dermatol Venereol Leprol 2018;84:573-577 |
Abstract
Background: Erythema nodosum leprosum is an immune-mediated complication of leprosy which causes significant morbidity. Biomarkers in the pathogenesis of erythema nodosum leprosum are not yet fully determined.
Aim: To determine macrophage migration inhibitory factor levels in the sera of leprosy patients with erythema nodosum leprosum and to correlate the same with clinical parameters.
Methods: This cross-sectional study included 37 consecutive leprosy patients with active erythema nodosum leprosum and 31 age- and sex-matched controls. Detailed clinical history and examination findings were recorded including the severity and frequency of erythema nodosum leprosum. Slit skin smears and histopathologic examination were done in all patients at baseline. Serum macrophage migration inhibitory factor levels were determined using an enzyme-linked immunosorbent assay.
Results: Most of our patients were males (78.4%) and suffering from lepromatous leprosy (27, 73%) with a mean initial bacillary index of 3.38 ± 1.36. Recurrent and chronic patterns of erythema nodosum leprosum were seen in 15 (40.5%) and 6 (16.3%) patients, respectively. Most (86.5%) of our patients presented with moderate to severe erythema nodosum leprosum. The mean serum macrophage migration inhibitory factor level was 21.86 ± 18.7 ng/ml among patients while it was 11.78 ± 8.4 ng/ml in the control group (P < 0.01). There were no statistically significant correlations of macrophage migration inhibitory factor levels with erythema nodosum leprosum frequency or severity.
Limitation: Serum macrophage migration inhibitory factor levels in leprosy patients with no erythema nodosum leprosum and in patients with other inflammatory and autoimmune conditions were not assessed. Hence, this study falls short of providing the predictive value and specificity of higher macrophage migration inhibitory factor concentrations in serum as a biomarker of erythema nodosum leprosum.
Conclusion: Macrophage migration inhibitory factor levels are elevated in erythema nodosum leprosum patients as compared to controls. A larger sample size and macrophage migration inhibitory factor gene polymorphism analysis will be needed to elucidate the role of this pro-inflammatory cytokine in erythema nodosum leprosum.
Introduction
Erythema nodosum leprosum is an immune-mediated complication of leprosy which presents with inflammatory skin nodules, involvement of multiple organ systems and often runs a protracted course.[1] Tumor necrosis factor-alpha and interleukin-6 present in reactional lesions induce immune cells (T-cells, monocytes, macrophages) to release macrophage migration inhibitory factor which might contribute to the perpetuation of erythema nodosum leprosum.[1] Identifying a biomarker which participates in the immunopathogenesis of erythema nodosum leprosum, might yield a valuable therapeutic target. Macrophage migration inhibitory factor is one such key mediator which has been found to be elevated in the peripheral blood and/or tissues in many inflammatory and autoimmune conditions such as rheumatoid arthritis, systemic lupus erythematosus, inflammatory bowel diseases, psoriasis, atherosclerotic vascular disease, proliferative primary glomerulonephritis and various neoplasms.[2] This study aimed to evaluate macrophage migration inhibitory factor levels in patients of erythema nodosum leprosum in comparison with age- and sex-matched controls.
Methods
Patients and controls
All consecutive consenting leprosy patients with erythema nodosum leprosum seen at the leprosy clinic of our institute from April 2012 to March 2013 were recruited for the study. The Institute Ethics Committee approved the study protocol (MS/1849/Res/506) and all participants gave written informed consent before enrollment.
The diagnosis of leprosy was confirmed by histopathology and slit skin smears. Detailed clinical history and examination findings were recorded in all participants. An episode of erythema nodosum leprosum was diagnosed if a patient developed multiple, tender, evanescent nodules with or without ulceration, usually associated with constitutional symptoms. Erythema nodosum leprosum was defined as “acute” if it was a single episode lasting for <24 weeks, “recurrent” if there was a second or subsequent episode occurring 4 weeks or more after stopping treatment for the erythema nodosum leprosum and “chronic” if occurring for 24 weeks or more during which the patient required erythema nodosum leprosum treatment continuously or where any treatment-free period was of 27 days or less.[3],[4] The severity of erythema nodosum leprosum was graded arbitrarily as mild, moderate or severe based on Ramu's score: a score of 1–2 was considered mild, 3–5 moderate and >6, severe.[5] The presence of any deformity and nerve function impairment was also recorded.
All patients were treated with the World Health Organization (WHO) multidrug therapy (MDT) and prednisolone for erythema nodosum leprosum. Patients with recurrent and chronic erythema nodosum leprosum also received clofazimine, pentoxifylline and colchicine. None of our patients were on thalidomide at the time of blood sampling for macrophage migration inhibitory factor.
Whole blood samples (3 ml) without any anticoagulant were obtained for macrophage migration inhibitory factor quantification from patients and age- and sex-matched controls
Laboratory evaluation
Serum was separated and stored at −20°C before processing. Serum macrophage migration inhibitory factor levels were determined using a human enzyme-linked immunosorbent assay (R&D Systems, Quantikine).
Statistical analysis
All calculations were two-sided and statistical analysis was carried using SPSS version 17 (Statistical Packages for the Social Sciences, Chicago, IL, USA). All data were presented as mean ± standard error. As the data were skewed, the association between clinical parameters and serum macrophage migration inhibitory factor levels was assessed using nonparametric Kruskal–Wallis test and Mann–Whitney test. P < 0.05 was considered to be statistically significant. Spearman's correlation coefficient was applied to measure correlation between severity of erythema nodosum leprosum and serum macrophage migration inhibitory factor levels.
Results
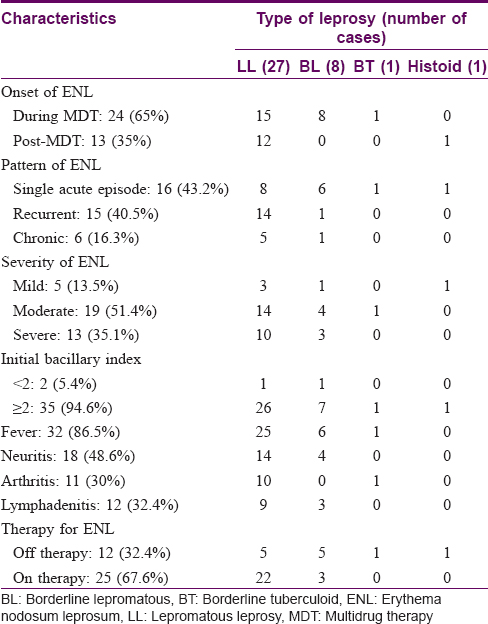
Thirty-seven patients of erythema nodosum leprosum and 31 age- and sex-matched controls were recruited. The mean age of the study population was 37.54 ± 12.9 years. Most of the patients were males (29, 78.4%) and 27 (73%) had lepromatous leprosy. Borderline lepromatous leprosy was diagnosed in 8 (21.6%) patients and borderline tuberculoid and histoid leprosy were diagnosed in 1 (2.7%) patient each. The mean initial bacillary index (BI) was 3.38 ± 1.36 [Table - 1]. The morphological index was positive in 9 (24.3%) patients and ranged from 1% to 10%. The duration of erythema nodosum leprosum in patients ranged from 2 days to 8 months. Twenty-four (65%) patients had their first episode of erythema nodosum leprosum during the course of multidrug therapy. Recurrent and chronic patterns of erythema nodosum leprosum were seen in 15 (40.5%) and 6 (16.3%) patients respectively. Thirty-two (86.5%) patients presented with moderate to severe erythema nodosum leprosum [Table - 1]. Neuritis was seen in 18 (49%) patients, while lymphadenitis and arthritis were seen in 12 (32%) and 11 (30%) patients respectively. There was no statistically significant correlation between the pattern or severity of erythema nodosum leprosum and the bacillary index (P > 0.05) [Table - 2].

Serum macrophage migration inhibitory factor values
The mean serum macrophage migration inhibitory factor level in the sera of erythema nodosum leprosum patients was 21.86 ± 18.7 ng/ml, while it was 11.78 ± 8.4 ng/ml in the control group (P < 0.05) [Figure - 1]. Mean serum macrophage migration inhibitory factor levels [Table - 3] were seen to be more elevated in borderline lepromatous patients (P - 0.458) and in patients presenting with chronic erythema nodosum leprosum (P - 0.878). Patients of erythema nodosum leprosum with initial bacillary index ≥2 showed higher mean serum macrophage migration inhibitory factor levels (P - 0.644). None of these other results were statistically significant [Figure - 2]. There was no significant difference in the serum macrophage migration inhibitory factor levels between patients on systemic corticosteroids and treatment-naïve patients.
 |
| Figure 1: Serum macrophage migration inhibitory factor levels in erythema nodosum leprosum patients versus controls. Summary: The macrophage migration inhibitory factor levels in the erythema nodosum leprosum patients were significantly higher than in the controls |

 |
| Figure 2: Scatter plot with linear regression analysis of correlation between serum macrophage migration inhibitory factor values and clinical severity of erythema nodosum leprosum. Summary: The scatterplot does not show any relationship of the serum macrophage migration inhibitory factor values with severity of erythema nodosum leprosum |
Discussion
Leprosy is not only a bacteriological disease but also an immunological disease with complex host-mycobacterial interactions which eventually determine the course of disease.[6] Erythema nodosum leprosum is a debilitating multisystem disorder which complicates leprosy and is a major cause of morbidity. Characterized by fever, malaise and painful erythematous cutaneous nodules, it occurs in approximately 10% of patients with borderline lepromatous leprosy and 50% of those with lepromatous leprosy.[3],[4],[7] Erythema nodosum leprosum is often recurrent or chronic, and frequently severe.[6]
Macrophage migration inhibitory factor was one of the first cytokines to be identified, almost 40 years ago, during studies of the delayed type hypersensitivity reaction. However its biological activities remained unclear until the cloning of human macrophage migration inhibitory factor complementary DNA.[8] In 1991, a search for new regulators of inflammation led to the rediscovery of macrophage migration inhibitory factor as a molecule released, similar to a hormone, by cells of the anterior pituitary gland after exposure to lipopolysaccharide endotoxin.[7] This intriguing observation indicated that macrophage migration inhibitory factor could be a mediator that links the endocrine and immune systems.[9] An emerging concept is that macrophage migration inhibitory factor has a central role as a regulator of innate immune and inflammatory responses, with implications for the development of new therapies in human sepsis and other inflammatory diseases.[10],[11]
Macrophage migration inhibitory factor normally circulates at basal levels in serum with additional secretion both from the anterior pituitary and by activated monocyte/macrophages and T-lymphocytes in response to various invasive stimuli.[9] Macrophage migration inhibitory factor is also released from macrophages that have been stimulated by glucocorticoids.[12] Glucocorticoid-induced macrophage migration inhibitory factor secretion follows a bell-shaped dose–response curve, decreasing at concentrations of dexamethasone ≥10−8 M. Once released, macrophage migration inhibitory factor overrides or antagonizes glucocorticoid suppression of macrophage cytokine (i.e., tumor necrosis factor α, interleukin-1, interleukin-6, interleukin-8) production in vitro, and endotoxin lethality in vivo.[13] The magnitude of this effect varies with the concentration of both glucocorticoid and macrophage migration inhibitory factor, suggesting that the two mediators act in a mutually counter-regulating manner to control cytokine production and inflammatory responses.[13] Thus, the expression of macrophage migration inhibitory factor, which antagonises the effects of glucocorticoids, is induced by glucocorticoids.
There are a few conflicting reports of macrophage migration inhibitory factor activity in leprosy patients in the literature.[14],[15],[16] Katz et al. evaluated the activity of macrophage migration inhibitory factor in patients of tuberculoid leprosy (good cell-mediated immunity) and lepromatous leprosy (poor cell-mediated immunity) and found higher production of macrophage migration inhibitory factor when exposed to lepromin in tuberculoid patients.[14] The authors concluded that the low production of macrophage migration inhibitory factor in lepromatous leprosy is due to lack of lymphocyte activation and delayed hypersensitivity reaction in the latter. Han et al. studied the ability of leprous lymphocytes to inhibit guinea pig macrophage and leprous macrophage migration.[15] Lymphocytes from lepromatous leprosy failed to inhibit the migration of guinea pig and leprous macrophages in contrast to lymphocytes from tuberculoid patients. These findings may indicate that the ability of lepromatous lymphocytes to secrete macrophage migration inhibitory factor is depressed while the ability of lepromatous macrophages to react to macrophage migration inhibitory factor is not affected. Rea and Yoshida in 1982 studied macrophage migration inhibitory factor activity in leprosy patients in reactional states.[16] Elevated macrophage migration inhibitory factor activity was found to be strongly associated with reactional states including active erythema nodosum leprosum. In the current study, we observed a statistically significant elevation in the macrophage migration inhibitory factor levels in erythema nodosum leprosum patients as compared to controls (P < 0.05). However, we were unable to find a statistically significant correlation between macrophage migration inhibitory factor and various aspects of erythema nodosum leprosum such as pattern, frequency and severity. This may be due to the high standard deviation from mean value of macrophage migration inhibitory factor levels in the erythema nodosum leprosum patients studied, which in turn might be due to the varying doses and durations of steroid therapy, a confounding factor in our analysis. However, serum macrophage migration inhibitory factor values were also elevated in patients who were not on any treatment for the erythema nodosum leprosum.
Serum macrophage migration inhibitory factor levels are low in patients of lepromatous leprosy due to a lack of lymphocyte activation.[14] It appears that the upregulation of cell-mediated immunity spontaneously or by treatment initiation leads to lymphocyte activation. The unregulated increased in cell-mediated immunity, evident by rise in the serum macrophage migration inhibitory factor levels, may precipitate erythema nodosum leprosum reactions. Thus, elevated macrophage migration inhibitory factor levels in sera of erythema nodosum leprosum patients could contribute to the formation of painful skin nodules by immunological activation.
It is an already known fact that an interaction between genetic susceptibility variants and immune system results in development of cutaneous inflammatory diseases. In this context larger prospective studies involving identification of macrophage migration inhibitory factor gene polymorphisms particularly in the promoter region of the gene, would be necessary to evaluate the impact of macrophage migration inhibitory factor on erythema nodosum leprosum susceptibility in leprosy patients. A limitation of our study is that serum macrophage migration inhibitory factor levels in leprosy patients without erythema nodosum leprosum were not assessed. This study also does not provide the predictive value and specificity of higher macrophage migration inhibitory factor concentrations in serum as a biomarker of erythema nodosum leprosum as controls with other inflammatory and autoimmune conditions were not included.
Conclusion
Serum macrophage migration inhibitory factor levels are elevated in erythema nodosum leprosum patients. Prospective studies with larger samples including patients from all categories of leprosy as well as macrophage migration inhibitory factor gene polymorphism analysis will be needed to understand the role of this pro-inflammatory cytokine in leprosy. Future studies could also evaluate serum macrophage migration inhibitory factor as a marker to predict the risk of erythema nodosum leprosum.
The study was supported by the Intramural Research Grant from Postgraduate Institute of Medical Education and Research.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
| 1. |
Kahawita IP, Lockwood DN. Towards understanding the pathology of erythema nodosum leprosum. Trans R Soc Trop Med Hyg 2008;102:329-37.
[Google Scholar]
|
| 2. |
Donn RP, Plant D, Jury F, Richards HL, Worthington J, Ray DW, et al. Macrophage migration inhibitory factor gene polymorphism is associated with psoriasis. J Invest Dermatol 2004;123:484-7.
[Google Scholar]
|
| 3. |
Pocaterra L, Jain S, Reddy R, Muzaffarullah S, Torres O, Suneetha S, et al. Clinical course of erythema nodosum leprosum: An 11-year cohort study in Hyderabad, India. Am J Trop Med Hyg 2006;74:868-79.
[Google Scholar]
|
| 4. |
Kumar B, Dogra S, Kaur I. Epidemiological characteristics of leprosy reactions: 15 years experience from North India. Int J Lepr Other Mycobact Dis 2004;72:125-33.
[Google Scholar]
|
| 5. |
Iyer CG, Ramu G. An open trial with clofazimine in the management of recurrent lepra reaction using thalidomide as a control drug. Lepr India 1976;48 4 Suppl: 690-4.
[Google Scholar]
|
| 6. |
Kumar B, Dogra S. Leprosy: A disease with diagnostic and management challenges! Indian J Dermatol Venereol Leprol 2009;75:111-5.
[Google Scholar]
|
| 7. |
Voorend CG, Post EB. A systematic review on the epidemiological data of erythema nodosum leprosum, a type 2 leprosy reaction. PLoS Negl Trop Dis 2013;7:e2440.
[Google Scholar]
|
| 8. |
Weiser WY, Temple PA, Witek-Giannotti JS, Remold HG, Clark SC, David JR. Molecular cloning of a cDNA encoding a human macrophage migration inhibitory factor. Proc Natl Acad Sci U S A 1989;86:7522-6.
[Google Scholar]
|
| 9. |
Calandra T, Roger T. Macrophage migration inhibitory factor: A regulator of innate immunity. Nat Rev Immunol 2003;3:791-800.
[Google Scholar]
|
| 10. |
Wu SP, Leng L, Feng Z, Liu N, Zhao H, McDonald C, et al. Macrophage migration inhibitory factor promoter polymorphisms and the clinical expression of scleroderma. Arthritis Rheum 2006;54:3661-9.
[Google Scholar]
|
| 11. |
Bernhagen J, Calandra T, Mitchell RA, Martin SB, Tracey KJ, Voelter W, et al. MIF is a pituitary-derived cytokine that potentiates lethal endotoxaemia. Nature 1993;365:756-9.
[Google Scholar]
|
| 12. |
Leech M, Metz C, Bucala R, Morand EF. Regulation of macrophage migration inhibitory factor by endogenous glucocorticoids in rat adjuvant-induced arthritis. Arthritis Rheum 2000;43:827-33.
[Google Scholar]
|
| 13. |
Calandra T, Bernhagen J, Metz CN, Spiegel LA, Bacher M, Donnelly T, et al. MIF as a glucocorticoid-induced modulator of cytokine production. Nature 1995;377:68-71.
[Google Scholar]
|
| 14. |
Katz SI, DeBetz BH, Zaias N. Production of macrophage inhibitory factor by patients with leprosy. Arch Dermatol 1971;103:358-61.
[Google Scholar]
|
| 15. |
Han SH, Weiser RS, Wang JJ, Tsai LC, Lin PP. The behavior of leprous lymphocytes and macrophages in the macrophage migration-inhibition test. Int J Lepr Other Mycobact Dis 1974;42:186-92.
[Google Scholar]
|
| 16. |
Rea TH, Yoshida T. Serum macrophage migration inhibition activity in patients with leprosy. J Invest Dermatol 1982;79:336-9.
[Google Scholar]
|
Fulltext Views
2,334
PDF downloads
1,713





