Translate this page into:
Stevens–Johnson syndrome-like reaction without mucosal lesions associated with cyclophosphamide
Correspondence Address:
Chun-An Yao
Department of Dermatology, Cathay General Hospital, No. 280, Sec. 4, Renai Road, Taipei 106
Taiwan
| How to cite this article: Lo Y, Yao CA. Stevens–Johnson syndrome-like reaction without mucosal lesions associated with cyclophosphamide. Indian J Dermatol Venereol Leprol 2019;85:101-103 |
Sir,
Stevens–Johnson syndrome is associated with significant morbidity and mortality. Stevens–Johnson syndrome caused by cyclophosphamide has been rarely reported.[1] Here we report a case of Stevens–Johnson syndrome-like reaction without mucosal lesions, induced by cyclophosphamide.
A 64-year-old man with no significant medical history presented to our department with denuded areas of skin since the last 2 days. One month before the development of the rash, he was referred for oliguria to our hospital, Cathay General Hospital, Taipei, Taiwan. Laboratory data demonstrated anemia (hemoglobin: 11.2 g/dl; normal >14) and mild thrombocytopenia (1.36 × 105 μl−1), with white blood cells within the normal range (5630 mm−3). Alanine and aspartate aminotransferase levels were slightly elevated (glutamic oxaloacetic transaminase/glutamic pyruvic transaminase: 57/57; normal <35 U/l). Impaired kidney function was evaluated with an elevated serum creatinine level (7.56 mg/dl; normal <1.5), elevated blood urine nitrogen (93 mg/dl; normal <20), proteinuria and microscopic hematuria (red blood cells: 50–99/hpf) were also present. Antinuclear antibody was negative. A normal hemoglobin A1c level excluded the diagnosis of diabetic nephropathy. Electrophoresis and antineutrophil cytoplasmic antibody were negative for systemic amyloidosis and antineutrophil cytoplasmic antibody-related vasculitis. The renal sonographic examination yielded increased kidney size and cortical echogenicity. Rapidly progressive glomerulonephritis was thus diagnosed, on ultrasound. Treatment was started with 30 mg prednisolone and furosemide 40 mg, followed by 100 mg cyclophosphamide daily starting 2 weeks thereafter. No antibiotic was administered as there was no evidence of infection. One week after institution of cyclophosphamide, an erythematous rash was noticed on the neck, alongwith intermittent fever up to 39.4°C. Subsequently, the lesions spread to the face, upper chest, upper abdomen and back within 2 days. The patient also complained of mild soreness on the upper back and generalised weakness. Physical examination revealed erythematous maculopapular lesions on the neck, upper chest and back [Figure - 1]a. The patient remained asymptomatic, except for mild discomfort over these skin lesions. There were no other systemic symptoms. Atypical target lesions and denuded skin were noted on the neck and face [Figure - 1]b. There were no lesions on the extremities, lower abdomen, oral mucosa and genitalia. Nikolsky's sign was positive. Epidermal detachment was observed over 10% of the body surface area. Biopsy from the upper back revealed full-thickness apoptosis of keratinocytes, lymphocyte exocytosis and superficial perivascular lymphocytic infiltration [Figure - 2]. Laboratory data demonstrated mild leukopenia (3660 mm−3; normal >4000). Differentially, segmented or mature neutrophils comprised 80.4%, and lymphocytes were 3.9%. Blood cultures for bacterial infection yielded negative results. Laboratory testing for mycoplasma pneumonia, cryptococcus, syphilis and human immunodeficiency virus were all negative. Screening for hepatitis B and C was also negative. Chest skiagram showed no abnormality. The diagnosis of Stevens–Johnson syndrome-like reaction was made, without evidence of any infection contributing to the presentation. We had considered acute phototoxic reaction as a diagnostic probability at the beginning. The most likely culprit which would have caused phototoxic reaction is furosemide, but the clinical course was not compatible with acute phototoxic reaction. The acute phototoxic reaction usually appears after 24 hours, and peaks at 48 to 72 hours. The cutaneous manifestations of our patient appeared after three weeks' administration of furosemide and one week administration of cyclophosphamide. And there is no previous report regarding cyclophosphamide causing acute phototoxic reaction. Besides, after admission to our hospital, the patient was not exposed to sunlight at all. Due to the above reasons, acute phototoxic reaction was not considered to be suitable for the diagnosis. Drug history was reviewed and scored according to the algorithm for assessment of drug causality in Stevens–Johnson syndrome (score 3 of ALDEN score). Treatment with 30 mg prednisolone daily was continued and cyclophosphamide was discontinued immediately. The lesions healed gradually with postinflammatory hyperpigmentation and desquamation [Figure - 3].
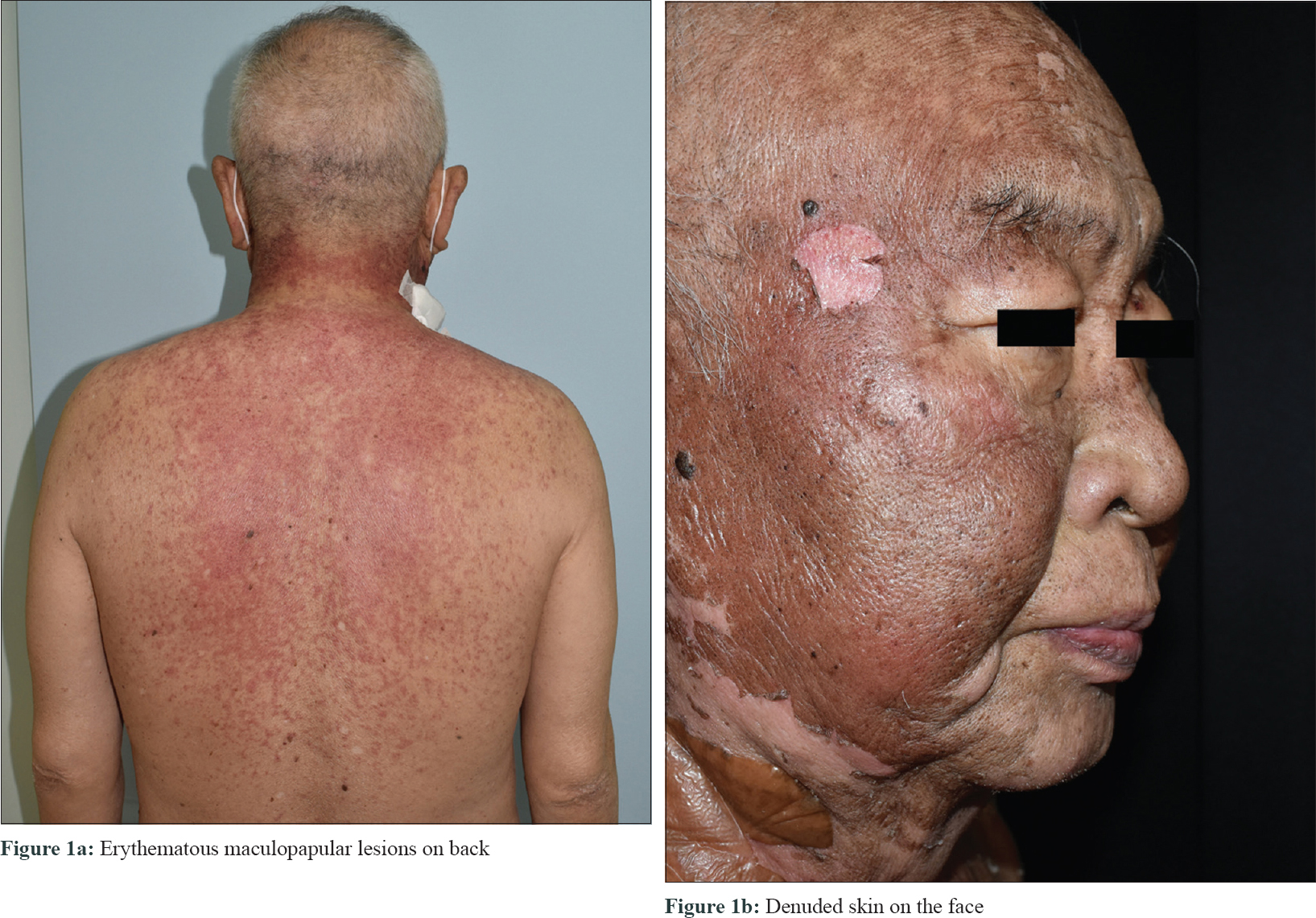 |
| Figure 1: |
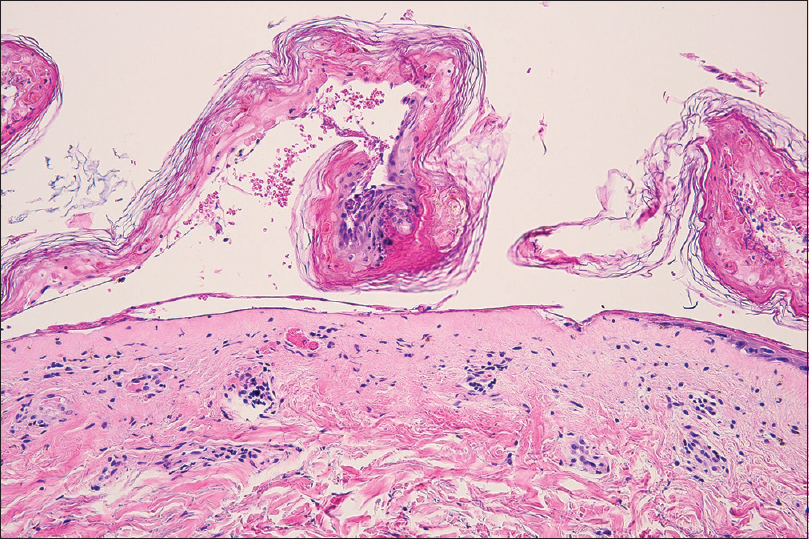 |
| Figure 2: Biopsy specimen showing full-thickness apoptosis of keratinocytes, lymphocyte exocytosis and superficial perivascular lymphocytic infiltration (hematoxylin and eosin, ×200) |
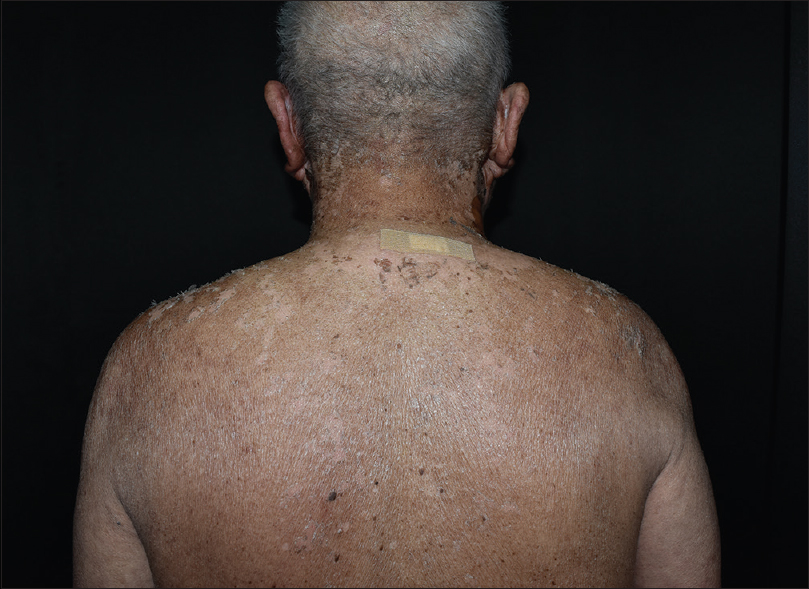 |
| Figure 3: The recovery of the skin following discontinuation of cyclophosphamide for 2 weeks. The lesions healed gradually with postinflammatory hyperpigmentation and desquamation |
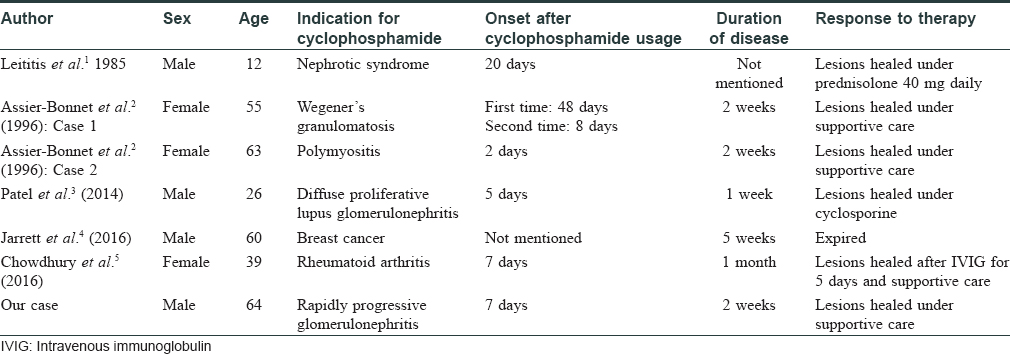
Cyclophosphamide, an antimetabolic and cytotoxic agent, is usually considered as a steroid-sparing medication. Its cutaneous side effects include anagen effluvium, pigmented bands on the teeth, acral erythema and diffuse hyperpigmentation of the skin and nails. Literature reviews show that few publications have discussed cyclophosphamide-induced Stevens–Johnson syndrome [Table - 1].[1],[2],[3],[4],[5] Two cases were previously described, both without mucosal involvement.[2] Combination therapy of docetaxel and cyclophosphamide was also noted to induce Stevens–Johnson syndrome in a man with breast cancer, recently.[4] Moreover, cyclophosphamide and mesna were also reported to induce toxic epidermal necrolysis in a patient with rheumatoid arthritis.[5] However, most patients with Stevens–Johnson syndrome have mucosal involvement. The clinical picture and pathology findings of our patient are consistent with Stevens–Johnson syndrome except for the absence of mucosal involvement. This reaction pattern is similar to previously described cases.[2] The underlying pathomechanism of mucosal sparing in these cases remains obscure, but the antiinflammatory properties of cyclophosphamide may lead to the atypical presentations. Owing to the limited number of cases, no predisposing factors could be found. Age distribution was wide, and incidence in both sexes was equally reported. Although cyclophosphamide has been used to treat Stevens–Johnson syndrome in a few case reports, clinicians should be aware that this immunosuppressive agent can also potentially evoke serious adverse reaction such as Stevens–Johnson syndrome/toxic epidermal necrolysis.
In conclusion, we report a case of Stevens–Johnson syndrome-like reaction without mucosal lesions possibly caused by cyclophosphamide. This case highlights the importance of taking into consideration cyclophosphamide as a possible culprit drug for causing Stevens–Johnson syndrome.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form, the patient has given his consent for his images and other clinical information to be reported in the journal. The patient understands that name and initials will not be published and due efforts will be made to conceal identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
| 1. |
Leititis JU, Burghard R, Rietschel E, Rieger CH, Brandis M. Stevens-Johnson syndrome during an immunosuppressive therapy with cyclophosphamide and prednisone. Klin Padiatr 1985;197:441-2.
[Google Scholar]
|
| 2. |
Assier-Bonnet H, Aractingi S, Cadranel J, Wechsler J, Mayaud C, Saiag P. Stevens-Johnson syndrome induced by cyclophosphamide: Report of two cases. Br J Dermatol 1996;135:864-6.
[Google Scholar]
|
| 3. |
Patel MP, Kute VB, Vanikar AV, Trivedi HL. Cyclophosphamide-induced toxic epidermal necrolysis: Vigilance needed. Clin Kidney J 2014;7:323-4.
[Google Scholar]
|
| 4. |
Jarrett B, Ghazala S, Chao J, Chaudhary S. Case of Steven-Johnson syndrome in a male with breast cancer secondary to docetaxel/cyclophosphamide therapy. BMJ Case Rep 2016;2016. pii: bcr2016217255.
[Google Scholar]
|
| 5. |
Chowdhury AC, Misra DP, Patro PS, Agarwal V. Toxic epidermal necrolysis due to therapy with cyclophosphamide and mesna. A case report of a patient with seronegative rheumatoid arthritis and rheumatoid vasculitis. Z Rheumatol 2016;75:200-2.
[Google Scholar]
|
Fulltext Views
4,651
PDF downloads
3,227





