Translate this page into:
Stevens-Johnson syndrome/toxic epidermal necrolysis induced by pirfenidone
Corresponding author: Dr. Dario de Perosanz-Lobo, Crta. Colmenar Viejo, Km 9100, Madrid-28034, Spain. dario.perosanz@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: de Perosanz-Lobo D, Fernández-Nieto D, Burgos-Blasco P, Aroca-Ruiz M, Fernández-Guarino M. Stevens-Johnson syndrome/toxic epidermal necrolysis induced by pirfenidone. Indian J Dermatol Venereol Leprol 2021;87:542-4.
Sir,
A 78-year-old woman presented with a 24 h history of a pruriginous cutaneous rash along with odynophagia and photophobia. She had a personal history of idiopathic pulmonary fibrosis (IPF) and had recently started therapy with pirfenidone (Esbriet®) two weeks ago. Besides pirfenidone, the patients' usual medications consisted of levothyroxine and pantoprazole as treatment for hypothyroidism and dyspepsia since many years. Physical examination showed an eruption of erythematous macules and papules with scarce incipient vesicles distributed over the patient’s trunk and proximal extremities. There was ophthalmological involvement with intense conjunctival hyperemia with pseudomembranes [Figure 1a]. Oral examination revealed extensive erosions on the buccal and palatal surfaces [Figure 1b]. The genital mucosa was not involved. Over the next few hours, the eruption evolved rapidly, becoming confluent. Dusky areas with epidermal detachment were observed [Figure 1c], while oral lesions extended to lingual and labial mucosae. A severe cutaneous adverse reaction to pirfenidone was suspected and a skin biopsy was performed. Histopathology revealed full-thickness epidermal necrosis with a minimal lymphocytic infiltrate in the dermis [Figure 2]. These findings were consistent with the diagnosis of Stevens-Johnson syndrome/toxic epidermal necrolysis (SJS/TEN) related to pirfenidone. The patient was promptly transferred to a burn center with life support measures, including contact isolation and topical antibiotic to avoid superinfection. Pirfenidone administration was immediately withdrawn and subcutaneous etanercept 25 mg twice a week was initiated. This treatment has demonstrated an increased survival rate when administered early in suspected Stevens-Johnson syndrome/ toxic epidermal necrolysis (SJS/TEN).1 However, in this case, extensive blisters developed later over the patient’s back and chest, resulting in epidermal detachment of 15–20% of the total body surface area [Figure 3]. The patient required a three-month stay in the ICU until complete resolution of both cutaneous and mucosal lesions [Figure 4]. Etanercept administration was maintained until the end of therapy. During that time, no other medication was administered (cyclosporine nor steroids).
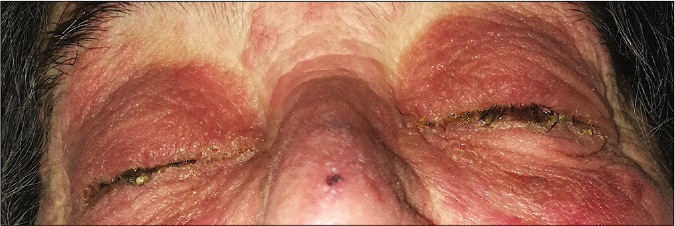
- Physical examination 48 h after the onset of skin symptoms. Prominent conjunctival involvement with profuse secretion and crusting
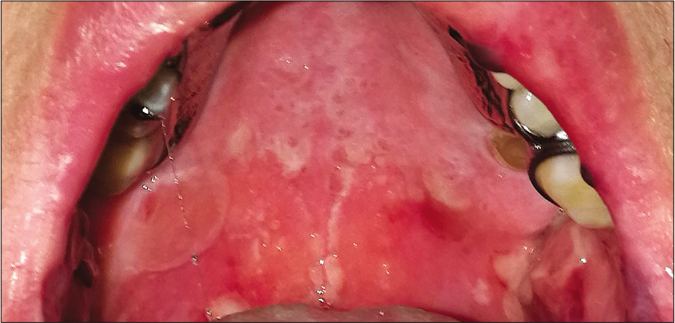
- Physical examination 48 h after the onset of skin symptoms. Extensive erosions on the palatal mucosa.
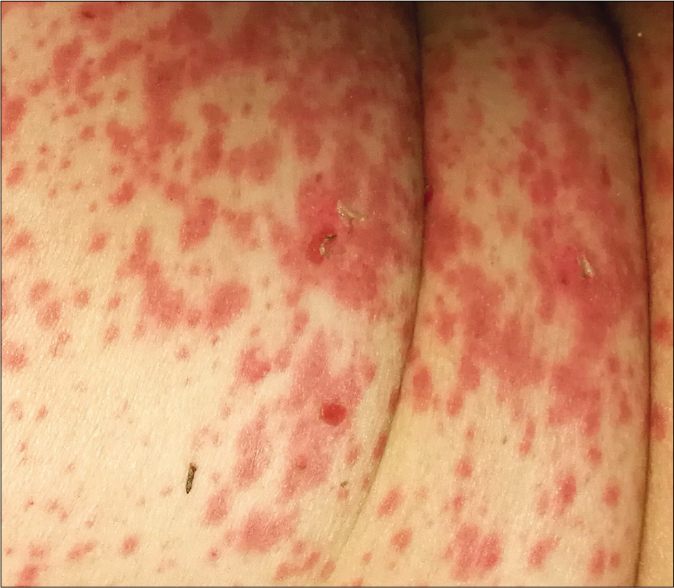
- Physical examination 48 h after the onset of skin symptoms. Close up view of atypical targetoid lesions on the trunk with scarce areas of epidermal detachment
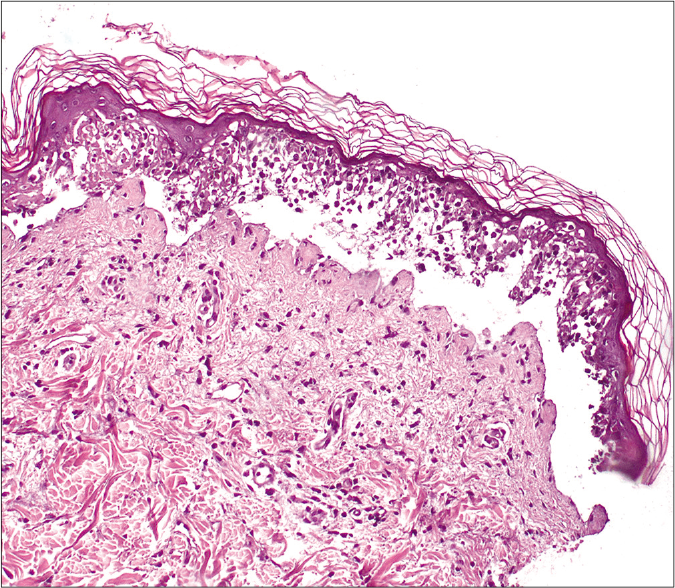
- Histopathological findings showing full-thickness epidermal necrosis with subepidermal detachment and minimal dermal inflammatory infiltrate (H&E, ×200)
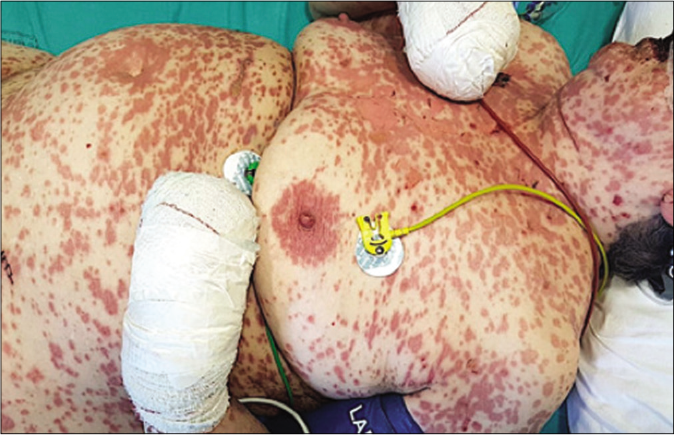
- Physical examination 96 h after the onset of skin symptoms. Epidermal detachment (Pseudo-Nikolsky sign) in approximately 15–20% of the total skin surface, consistent with the diagnosis of Stevens-Johnson syndrome/ toxic epidermal necrolysis (SJS/TEN)
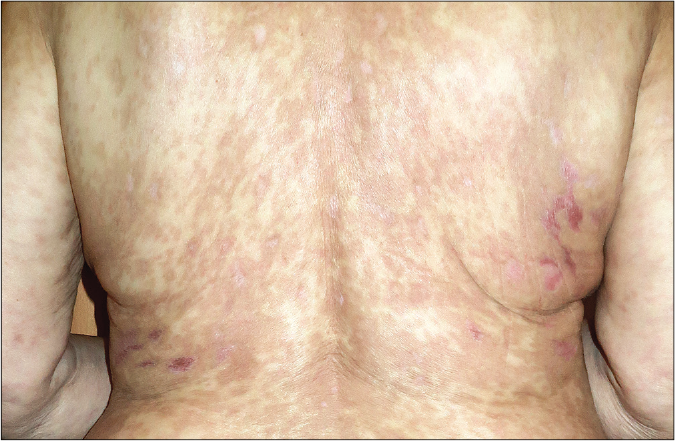
- Post-resolution image. Note the post-inflammatory hyperpigmentation
Idiopathic pulmonary fibrosis (IPF) is a chronic, progressive, fibrotic lung disease of unknown etiology. Despite its variable evolution, the median survival is only 2–3 years after the onset of symptoms. Pirfenidone is a novel orally administered pyridine with combined antifibrotic, antioxidant and anti-inflammatory actions. It has shown to reduce the decline in lung function and to prolong progression-free survival in these patients.2 Although the precise mechanism of action is unknown, it is thought to inhibit TGF-β and TNF-α and to reduce fibroblast proliferation and collagen synthesis.2,3 The most common adverse effects of pirfenidone include gastrointestinal symptoms, hepatic dysfunction and skin reactions. Among the latter, both “photosensitivity” and “skin rash” are listed as frequent side effects. Skin rash occurred in 11.6–32.2% of patients in different cohort studies and clinical trials, while photosensitive reactions were observed in 5.8– 12.2% of patients. Together photosensitivity and rash led to discontinuation of the drug in around 1–2%.4,5 However, little is known about categorization of this “rash” and “photosensitivity” reactions, since dermatological assessment has not been elaborated in the cited studies. TNF-α expression has been found to be elevated in plasma of Stevens-Johnson syndrome/ toxic epidermal necrolysis (SJS/TEN) patients. This molecule seems to be key in the pathogenesis of this condition, since it can augment the granulysin expression which is the main mediator for keratinocyte detachment of the epidermis. Etanercept attenuates granulysin expression through blockade of the TNF-α associated pathway, being shown to be an effective treatment in Stevens-Johnson syndrome/ toxic epidermal necrolysis (SJS/TEN).1 Although the final evolution of our patient was favorable, she required a prolonged stay in the ICU for a complete recovery. Whether this could have been shortened if alternative options such as cyclosporine or steroids were adopted is a question to be considered, although it is also possible that, without the etanercept, the evolution to TEN would have been unstoppable.
In conclusion, we present a case of Stevens-Johnson syndrome/ toxic epidermal necrolysis (SJS/TEN) induced by pirfenidone. Dermatologists need to be aware that pirfenidone can be associated with potentially life-threatening skin reactions, since there has recently been an increase in its prescription. Cutaneous adverse effects related to this drug need also to be better characterized in further studies.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- Randomized, controlled trial of TNF-α antagonist in CTL-mediated severe cutaneous adverse reactions. J Clin Invest. 2018;128:985-96.
- [CrossRef] [PubMed] [Google Scholar]
- Pirfenidone in patients with idiopathic pulmonary fibrosis (CAPACITY): Two randomised trials. Lancet. 2011;377:1760-9.
- [CrossRef] [Google Scholar]
- An open-label study of the long-term safety of pirfenidone in patients with idiopathic pulmonary fibrosis (RECAP) Respiration. 2017;94:408-15.
- [CrossRef] [PubMed] [Google Scholar]
- Long-term safety of pirfenidone: Results of the prospective, observational PASSPORT study. ERJ Open Res. 2018;4:084-2018.
- [CrossRef] [PubMed] [Google Scholar]





