Translate this page into:
Successful correction of pseudosyndactyly in recessive dystrophic epidermolysis bullosa using full thickness skin graft in resource-poor settings
2 Department of Dermatology, Venereology and Leprology, Postgraduate Institute of Medical Education and Research, Chandigarh, India
Correspondence Address:
Rahul Mahajan
Department of Dermatology, Venereology and Leprology, Postgraduate Institute of Medical Education and Research, Chandigarh - 160 012
India
| How to cite this article: Tripathy S, Dabas G, Handa S, De D, Mahajan R. Successful correction of pseudosyndactyly in recessive dystrophic epidermolysis bullosa using full thickness skin graft in resource-poor settings. Indian J Dermatol Venereol Leprol 2019;85:426-428 |
Sir,
In patients with recessive dystrophic epidermolysis bullosa, hand deformities cause serious impact on psychomotor and social development, as well as the quality of life.[1],[2] Many a times they face social isolation too.[1] Most surgeons are reluctant to operate on patients with epidermolysis bullosa because of the lack of awareness about the disease, high risk of recurrence, and non-availability of artificial skin grafts. We demonstrate the significant benefit following surgical correction of cocoon-like or mitten deformity in a boy with recessive dystrophic epidermolysis bullosa done in a relatively resource poor setting.
A 5-year-old boy presented to our outpatient department with persistent, widespread, traumatic blisters all over the body and functional disability of both the hands. He was small for his age (weight and height below 3rd percentile). He had erosions and bullae along with scarring and milia on many areas on the body. Both hands showed flexion contractures of all the fingers, adduction contracture of the thumb, pseudosyndactyly and anonychia [Figure - 1]. He also had scarring alopecia of the scalp.
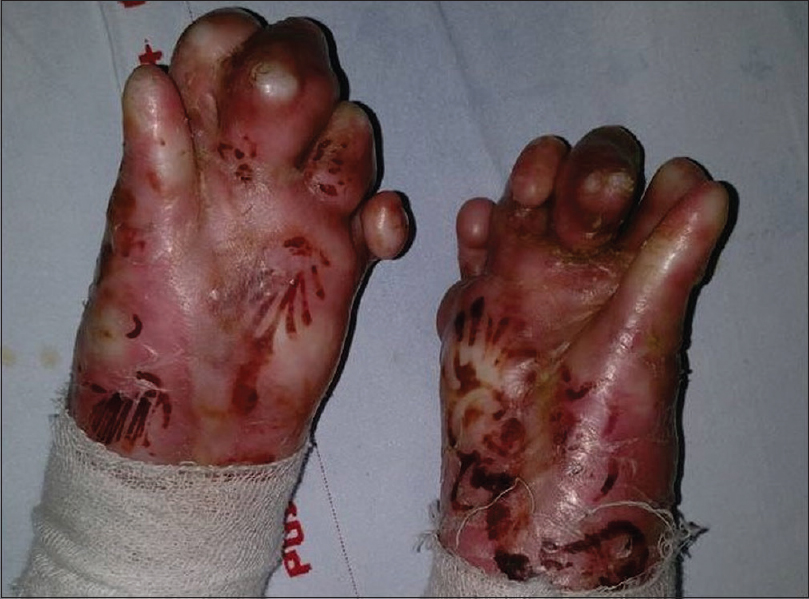 |
| Figure 1: Preoperative image showing mitten like deformity of both the hands |
Systematic analysis of the whole exome data revealed a compound heterozygous mutation p.R2777* and p.G1667E in the COL7A1 gene (reported elsewhere as novel variants). The variants were verified by capillary sequencing in the family. One hand was operated during each visit so as not to hamper the child's day to day activity and to reduce the discomfort due to prolonged general anaesthesia. A tourniquet with cotton padding was applied around the left upper arm to achieve a bloodless field. The contractures in the finger tips were released and stay sutures of 3-0 silk were passed through that area to prevent further injuries to the de-epithelialized surfaces. Thereafter, incisions were put to release the soft tissue contractures in the flexural aspects of interphalangeal and metacarpophalangeal joints taking care not to injure the flexor tendons and neurovascular structures [Figure - 2]. The raw areas were covered with a full-thickness skin graft obtained from apparently unaffected areas of the thigh. Primary closure of the donor site was done in layers with delayed absorbable suture 4-0 vicryl rapide (Polyglactin 910) and the graft site with 5-0 vicryl rapide. The fingers were immobilized with number one Kirschner wires, and wet collagen was applied on the remaining de-epithelialized surfaces. This was followed by a dressing with sofra-tulle gauze, soft tissue padding and plaster of Paris volar slab for immobilization. All possible precautions to prevent injuries were ensured during anaesthesia and trachea was atraumatically intubated and extubated successfully. Dressing was changed on the 7th postoperative day under general anesthesia to ensure careful handling of the grafts and to minimise associated pain. On that day the plaster of Paris volar slab was replaced with a thermoplastic slab. The Kirschner wires were removed after 2 weeks and physiotherapy, including range of motion exercises, was started once complete re-epithelialization was achieved. A similar procedure was repeated on the right hand after four months. Now one year after the first surgery, the child is able to eat, write and perform other important activities of daily living notably without any recurrence [Figure - 3]. The donor area also has healed well with scar formation.
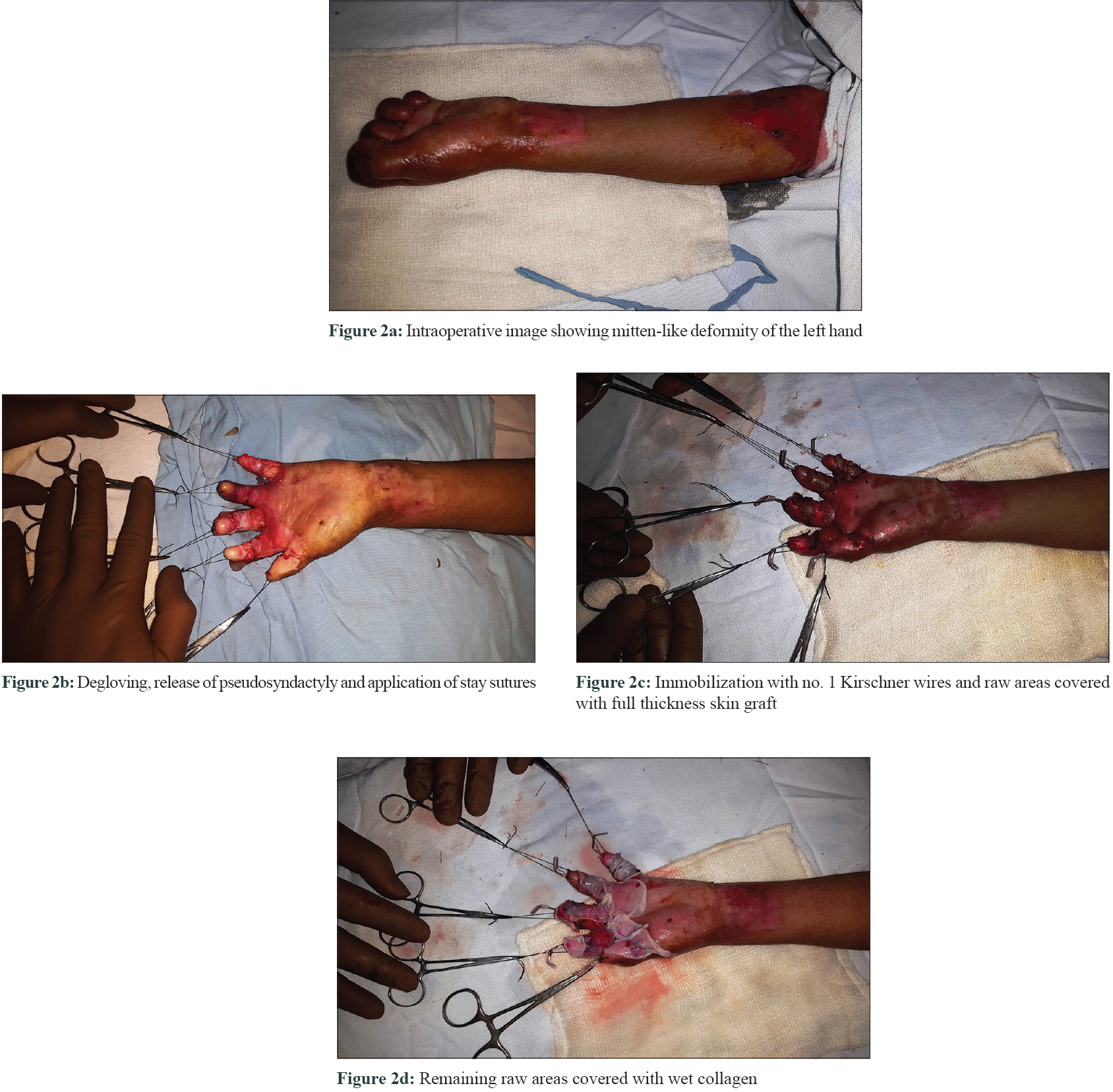 |
| Figure 2: |
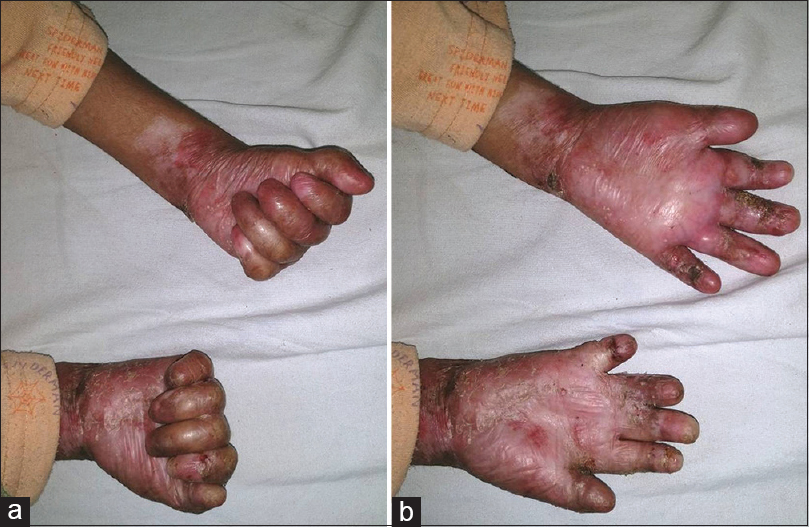 |
| Figure 3: (a and b) Postoperative images 1 year after both surgeries showing full range of movements of both the hands after second surgery |
Ideally, the surgery on patients with epidermolysis bullosa should be performed after optimizing the dermatological, medical and nutritional components and at a time when they can have regular postoperative occupational therapy. The treatment of hand deformity is a three-step approach involving degloving, closed capsulotomy and covering the wound with autologous or synthetic materials.[3] Different methods that have been used to deal with soft tissue defects include no coverage, split skin grafts, full thickness skin grafts, cultured keratinocytes and cellular allograft dermal matrix.[4] Usually full thickness skin grafts provide a stable tissue cover with less secondary contractions as they tend to grow with age. But as the skin stretches less easily in patients with dystrophic epidermolysis bullosa, grafts must completely fit the defect. Tissue-engineered skin grafts like epidermal keratinocyte grafts, autologous composite cultured skin grafts and acellular dermal allografts are scarcely available and expensive too.[5],[6] Providing anaesthesia is also challenging in patients with this disease. Blood pressure cuffs and face masks should be used with appropriate padding and lubrication. Upper airway devices must be avoided because there is a risk of intraoral bullae formation and hemorrhage. Even though endotracheal intubation is generally safe,[7] fiberoptic tracheal intubation will be more atraumatic.[8]
Long-term results of hand surgery in patients with epidermolysis bullosa show that recurrence of contractures is common and most of them occur within two to five years. Postoperative splinting, web-space protecting gloves and exercises must be continued lifelong to reduce recurrence.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form, the patient has given his consent for his images and other clinical information to be reported in the journal. The patient understand that name and initials will not be published and due efforts will be made to conceal identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
| 1. |
Fine JD, Johnson LB, Weiner M, Stein A, Cash S, Deleoz J,et al. Pseudosyndactyly and musculoskeletal contractures in inherited epidermolysis bullosa: Experience of the national epidermolysis bullosa registry, 1986-2002. J Hand Surg Br 2005;30:14-22.
[Google Scholar]
|
| 2. |
Eismann EA, Lucky AW, Cornwall R. Hand function and quality of life in children with epidermolysis bullosa. Pediatr Dermatol 2014;31:176-82.
[Google Scholar]
|
| 3. |
Greider JL Jr., Flatt AE. Care of the hand in recessive epidermolysis bullosa. Plast Reconstr Surg 1983;72:222-8.
[Google Scholar]
|
| 4. |
Ciccarelli AO, Rothaus KO, Carter DM, Lin AN. Plastic and reconstructive surgery in epidermolysis bullosa: Clinical experience with 110 procedures in 25 patients. Ann Plast Surg 1995;35:254-61.
[Google Scholar]
|
| 5. |
Phillips J, Rockwell WB. Surgical treatment of recessive dystrophic epidermolysis bullosa in the hand: Use of tissue-engineered skin (Apligraf). Ann Plast Surg 2003;50:441-2.
[Google Scholar]
|
| 6. |
Ozkaya O, Oreroǧlu AR, Akan M. The use of acellular dermal matrix in treatment of mitten hand in epidermolysis bullosa patients. J Hand Microsurg 2013;5:46-7.
[Google Scholar]
|
| 7. |
Boschin M, Ellger B, van den Heuvel I, Vowinkel T, Langer M, Hahnenkamp K. Bilateral ultrasound-guided axillary plexus anesthesia in a child with dystrophic epidermolysis bullosa. Paediatr Anaesth 2012;22:504-6.
[Google Scholar]
|
| 8. |
Bowen L, Burtonwood MT. Anaesthetic management of children with epidermolysis bullosa. BJA Educ 2018;18:41-5.
[Google Scholar]
|
Fulltext Views
4,308
PDF downloads
1,814





