Translate this page into:
The comparative efficacy and safety of azathioprine vs methotrexate as steroid-sparing agent in the treatment of airborne-contact dermatitis due to Parthenium
Correspondence Address:
Sanjeev Handa
Department of Dermatology, Venereology and Leprology, Postgraduate Institute of Medical Education and Research, Chandigarh
India
| How to cite this article: De D, Sarangal R, Handa S. The comparative efficacy and safety of azathioprine vs methotrexate as steroid-sparing agent in the treatment of airborne-contact dermatitis due to Parthenium. Indian J Dermatol Venereol Leprol 2013;79:240-241 |
Sir,
Airborne-contact dermatitis (ABCD) caused by the weed Parthenium hysterophorus is a chronic disorder, management of which is a therapeutic challenge. To maintain clinical remission, prolonged courses of systemic corticosteroids are required. However these are associated with many unacceptable side effects. Thus, steroid-sparing immunosuppressive agents are used in combination with corticosteroids. There are case reports/series on the use of azathioprine and methotrexate in the treatment of ABCD, but none of these reports have compared their efficacy and safety. The objective of this study was to compare the efficacy and safety of azathioprine vs methotrexate as steroid-sparing agent in the treatment of extensive ABCD secondary to parthenium.
A randomized trial was conducted between March 2010 and December 2010. Thirty adult patients (above the age of 18 years) of ABCD suspected to be secondary to parthenium and later confirmed on patch test, with clinical severity score (CSS) [1] >30, were recruited in the study. The CSS was based upon four parameters, namely erythema, itching, type of lesions, and area involved. Baseline routine investigations were done that included complete hemogram, liver function tests, renal function tests, chest X-ray, and urine analysis. Patients with abnormal baseline investigations were excluded.
Patients were randomly assigned to treatment with azathioprine 100 mg daily with oral corticosteroids in Group A and methotrexate 15 mg/week with oral corticosteroids in Group B. Prednisolone was initiated at the dose of 0.75 mg/kg/day and the dose was decreased by 10 mg every 2 weeks once >75% reduction in CSS was achieved. In the case of exacerbation the prevailing dose of prednisolone was increased by 10 mg and then tapered slowly. The patients were evaluated fortnightly for first 2 months and then monthly for 4 months, for the assessment of dermatitis severity by CSS and side effects, if any. Hematological and biochemical evaluations were done every 2 weeks for first 2 months and then monthly. The time taken for >75% reduction in CSS in each group was compared. The cumulative dose of oral prednisolone in 6 months of study period was calculated in both the groups and compared. All comparisons were put to statistical analysis by t-test using computer software: two sample assuming unequal variance.
Seventeen male and thirteen female patients (mean age 52.42 years) were included. The mean duration of disease in Group A patients was 7.3 ± 5.6 years (2-20 years) and 8.6 ± 1.73 years (1.5-15 years) in Group B patients. Out of 15 patients in each group, 12 patients in Group A and 14 patients in Group B completed the study. The mean initial CSS in Group A was 60.3 ± 13.34 (35.5-74.1) and in Group B 58.45 ± 13.34 (36.4-83.4), respectively. The mean time taken for >75% reduction in CSS was 9.5 ± 3.2 weeks (6.3-16 weeks) in Group A and 5.6 ± 1.3 weeks (4-8.6 weeks) in Group B [Figure - 1]a, b and [Figure - 2]a, b. This difference was statistically significant (P = 0.001). The mean dose of prednisolone in Group A was 1329.6 ± 231.66 mg (980-1740 mg) and in Group B was 1202.86 ± 97.74 mg (990-1690 mg), the difference was statistically not significant (P = 0.13). Six patients in Group A showed complete clearance of the disease within the study period and were on tapering doses of azathioprine while the rest six patients showed sustained control of the dermatitis. Azathioprine was tapered as 50 mg every 2 weeks in patients who showed complete clearance. Similarly, six patients in Group B showed complete clearance of the dermatitis and were on tapering doses of methotrexate (tapered as 5 mg every 2 weeks) while the remaining eight patients showed sustained relief.
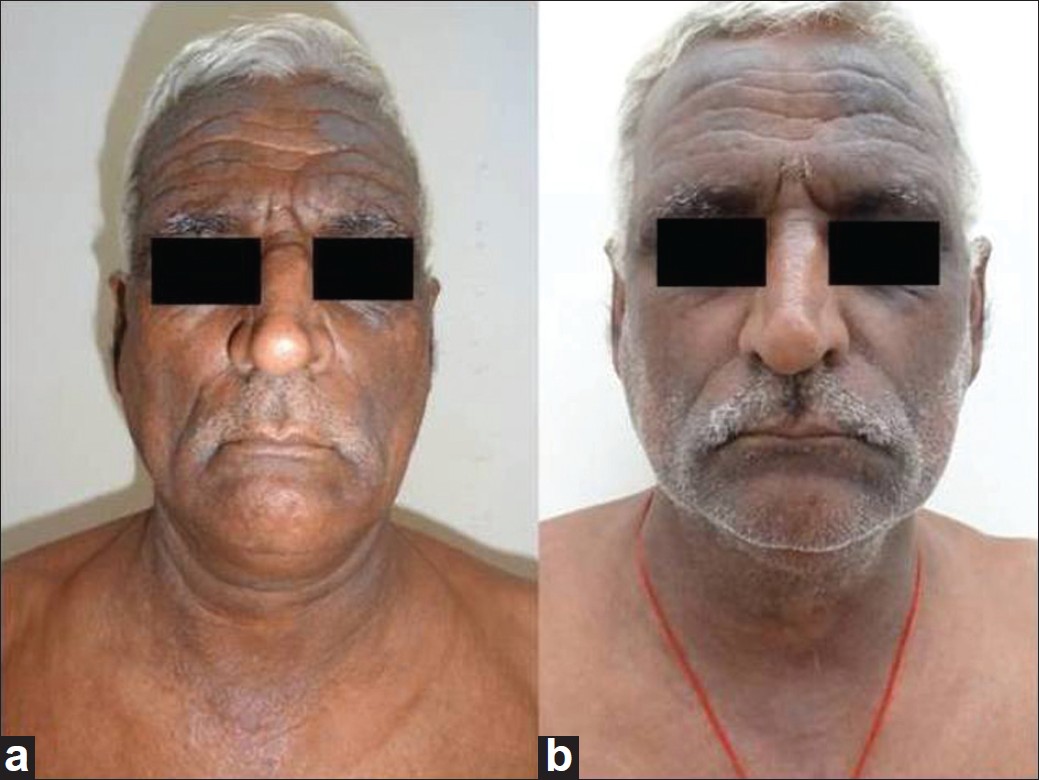 |
| Figure 1: (a) Pre-treatment (Group A), in a patient with ABCD in mixed pattern. (b) Eight weeks on treatment, Group A |
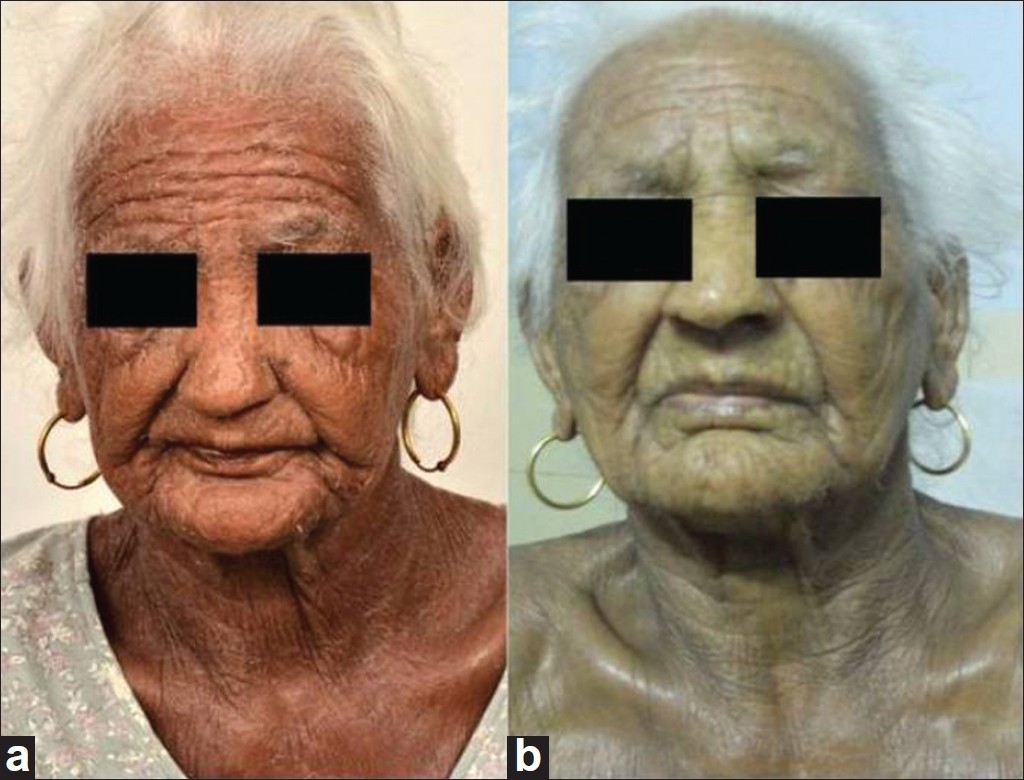 |
| Figure 2: (a) Pretreatment, Group B. (b) Four weeks on treatment, Group B |
Side effects in both groups were minimal. It included herpes zoster (1), tinea corporis (1) and folliculitis (2) in Group A. In Group B; tinea corporis (1) and folliculitis (3) were seen along with mild transaminitis (1), which did not require discontinuation of methotrexate.
In previous studies, the efficacy of azathioprine has been established. [1],[2],[3],[4] Excellent control of dermatitis in 95% of patients with azathioprine alone has been described within 3.6 months of initiation of therapy. [4] In a recent systematic review, evidence of moderate quality was found for efficacy of azathioprine in ABCD to parthenium. [5] In our study, azathioprine was given along with systemic corticosteroids in tapering doses in the initial phase and >75% reduction in CSS was seen in mean time period of 9.5 weeks. The beneficial effect of methotrexate in parthenium dermatitis studied previously showed 50% improvement in dermatitis within 1 month of initiation of therapy with a sustained decrease in the dermatitis score with continuation of methotrexate for 6 months. [6] Our study, also shows >75% reduction in CSS within 5.6 weeks.
Comparing the cost of therapy in both groups, there was a significant difference, as one week therapy in the azathioprine group cost between INR 102 - INR 215, whereas in the methotrexate group this cost was reduced to INR 18 - INR 33 per week to the patient. (Retail prices of drugs in Indian pharmaceutical market, March 2012.)
In conclusion, though both azathioprine and methotrexate are effective and safe steroid-sparing agents in the treatment of parthenium-induced ABCD, methotrexate helps in achieving an earlier control of the dermatitis and is a cheaper alternative to azathioprine.
| 1. |
Verma KK, Bansal A, Sethuraman G. Parthenium dermatitis treated with azathioprine weekly pulse doses. Indian J Dermatol Venereol Leprol 2006;72:24-7.
[Google Scholar]
|
| 2. |
Sharma VK, Chakrabarti A, Mahajan V. Azathioprine in the treatment of parthenium dermatitis. Int J Dermatol 1998;37:299-302.
[Google Scholar]
|
| 3. |
Verma KK, Manchanda Y, Pasricha JS. Azathioprine as a corticosteroid sparing agent for the treatment of dermatitis caused by the weed parthenium. Acta Derm Venereol 2000;80:31-2.
[Google Scholar]
|
| 4. |
Verma KK, Mahesh R, Srivastava P, Ramam M, Mukhopadhyaya AK. Azathioprine versus betamethasone for the treatment of parthenium dermatitis: A randomised controlled study. Indian J Dermatol Venereol Leprol 2008;14:453-7.
[Google Scholar]
|
| 5. |
Schram ME, Borgonjen RJ, Bik CM, van der Schroeff JG, van Everdingen JJ, Spuls PI, et al. Off-label use of azathioprine in Dermatology: A systematic review. Arch Dermatol 2011;147:474-88.
[Google Scholar]
|
| 6. |
Sharma VK, Bhat R, Sethuraman G, Manchanda Y. Treatment of Parthenium dermatitis with methotrexate. Contact Dermatitis 2007;57:118-9.
[Google Scholar]
|
Fulltext Views
3,838
PDF downloads
1,321





